Attention-deficit/hyperactivity disorder (ADHD) is a neuropsychiatric disorder first presenting in childhood (before age 12) with core symptoms of hyperactivity, restlessness, impulsivity, and inappropriate attention (2). The disorder persists into adulthood in a considerable number of patients, with a worldwide prevalence of ADHD in adults at 1-4%. Genetic and environmental factors contribute to the disorder, and the microbiome is increasingly investigated for its potential role (3).
Approximately 9.4% of children in the US aged 4-17 have been diagnosed with ADHD as of 2016 (4). In Australia, as of 2015, 7.4% of children and adolescents fulfilled the criteria for ADHD. The prevalence of ADHD was lower in females aged 12–17 than those aged 4-11 (2.7% vs 5.4%) but about the same for males (9.8% vs 10.9%) (5).
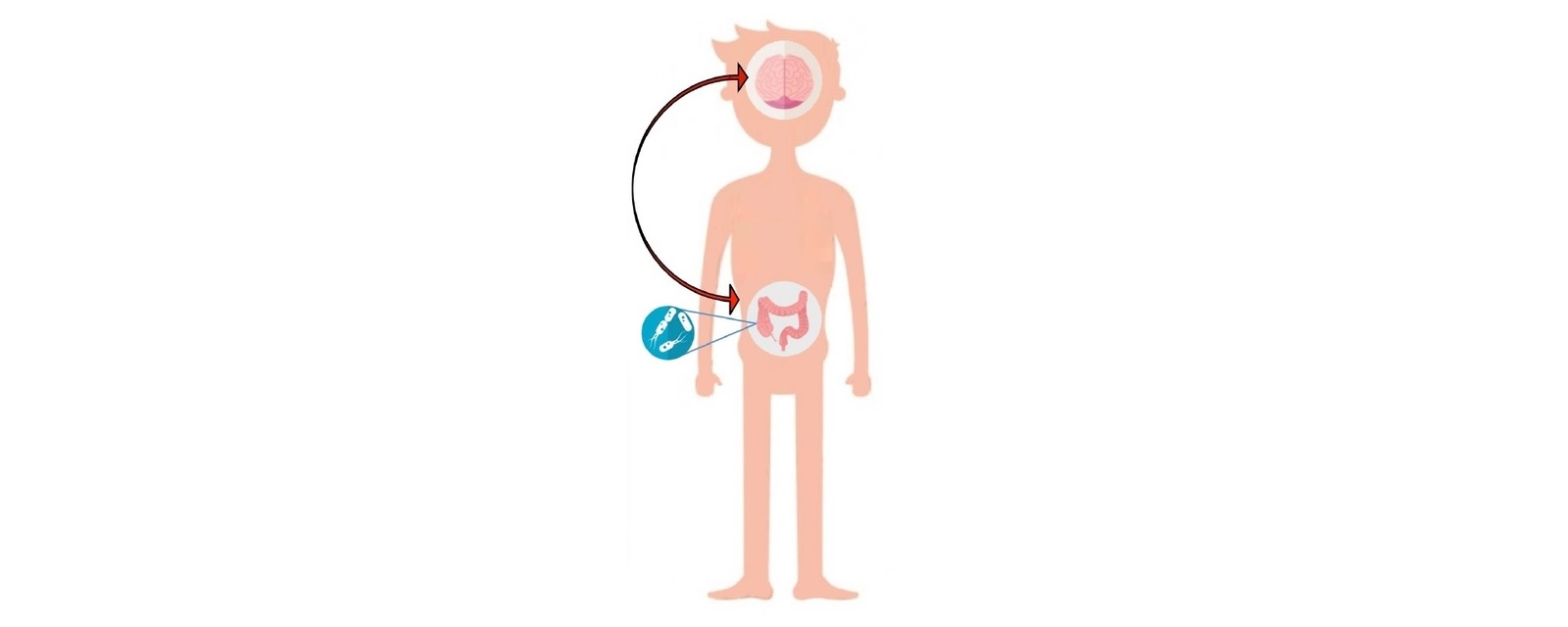
Increasing evidence suggests that the gut microbiota plays a key role in the gut-brain communication axis by influencing metabolism, inflammation, the hypothalamic-pituitary-adrenal axis and neurotransmission (6). Preclinical research shows promising results in targeting the microbiota, particularly during critical microbial-neural developmental windows, with the potential to prevent later neurodevelopmental deficits (7).
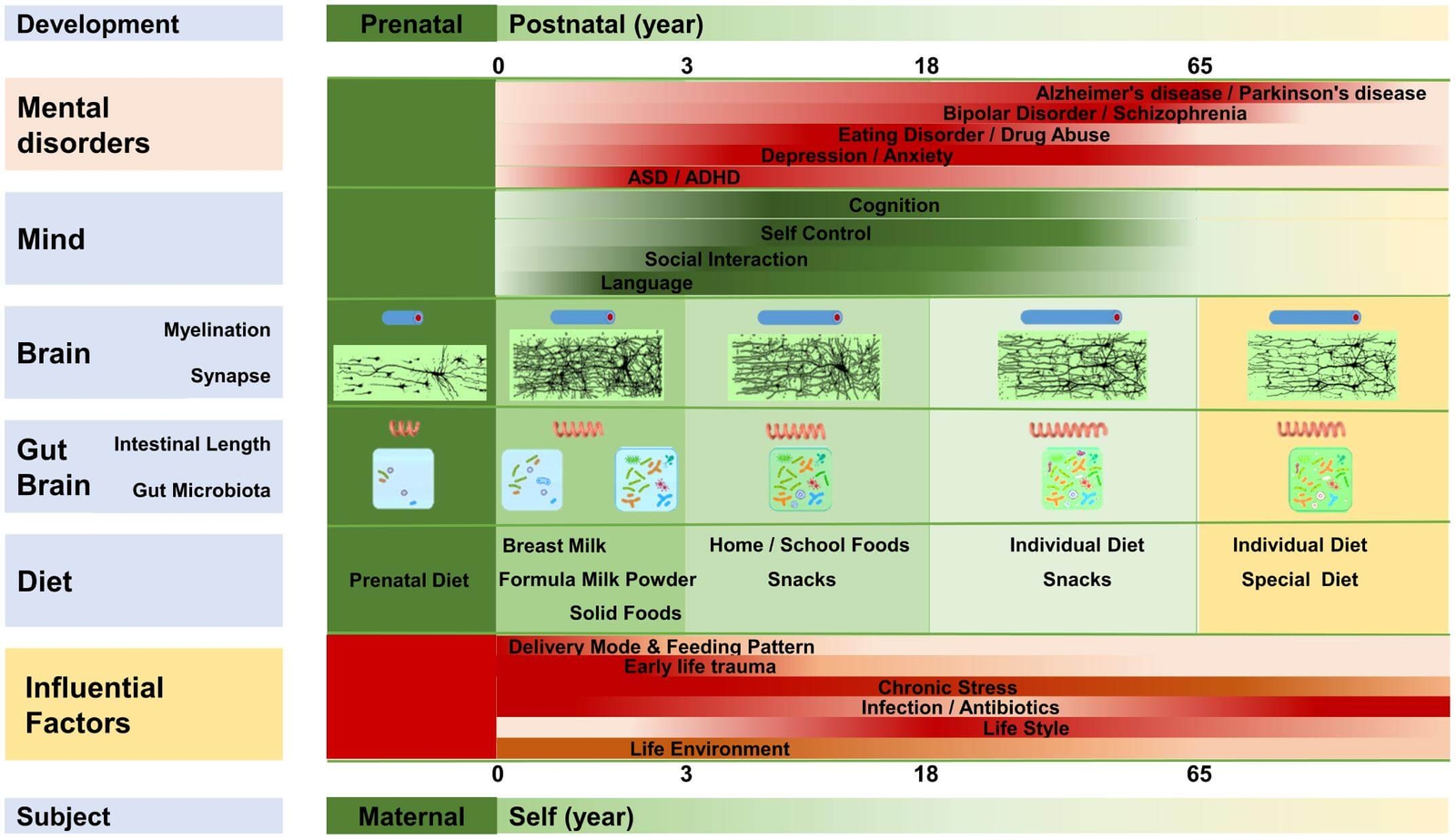
A prevailing hypothesis suggests that an unfavourable infant gut microbiota can affect brain development through epigenetic mechanisms (8,9). However, no direct clinical evidence supports a relationship between gut microbiota composition and ADHD symptoms (10). Rather, evidence of the role of the microbiome in ADHD is supported by a number of theoretical hypotheses as outlined below. This is emerging into a novel integrative model explaining the aetiology and pathogenesis of ADHD in a microbiota-gut-brain context. In this model, an imbalanced, less rich and/or diverse gut microbiota early in life is a core developmental feature of metabolic, immunologic, cognitive, emotional and behavioural symptoms in ADHD (11). Figure 1 highlights the link between early life microbiota disruption and ADHD development.
Figure 1. Microbiota disruption at different life stages and the incidence of different neuropsychiatric disorders (12) CC BY 4.0
Microbiome composition in ADHD
Gut microbiome studies in ADHD are in their early days. Initial evidence indicates that there may be a difference in microbiota composition early in life between children, adolescents and adults with ADHD and healthy controls (13,14,15).
A study found that children diagnosed with ADHD had decreased levels of Bifidobacterium longum early in life. The same study found that administration of Lactobacillus rhamnosus GG during the first 6 months of life may reduce the risk of ADHD. Despite what seems to be a protective effect of the probiotic, it did not result in significant effects on the microbiota composition (13).
A recent study found an abundance of Faecalibacterium was negatively associated with parental reports of ADHD symptoms in children. No significant difference in microbiome diversity was found however between the ADHD and control groups (6).
If the effect of microbiota composition early in life on ADHD proves to be correct (replicated), major functions of the gut microbiome will be affected (16):
- Protection from colonisation of pathogenic bacteria strains
- Strengthening of the intestinal barrier and limiting the penetration of bacteria and toxic content into the body
- Increasing efficiency of nutrient absorption
- Guiding the function and maturation of the immune system
Environmental risk factors
There is strong evidence that industrial chemicals such as toluene, lead, fluoride and methylmercury are neuro-developmentally toxic and capable of causing permanent brain damage at low levels of exposure during sensitive developmental stages (17). Childhood exposure to lead and prenatal exposure to organophosphates has been associated with ADHD although further evaluation is needed (18). Chemicals are able to cross the blood-brain barrier and be transported to the central nervous system. If barrier functions are reduced in ADHD then it is plausible that there will be increased sensitivity to toxic environmental exposure, as well as to bacterial toxins and food substances such as gluten, which shows endotoxin-mimicking and thus induces inflammation (19,20). It has been proposed that low-grade systemic inflammation may lead to the gradual destruction of the blood-brain barrier and possibly the neuroinflammation seen in ADHD (10,21). There is preliminary evidence of elevated pro-inflammatory cytokines in children and adolescents with ADHD (22).
Neurotransmitter synthesis
One proposed mechanism for the effects of gut microbiota on brain and behaviour is through their ability to synthesize neurotransmitter chemicals and their precursors (23). Clinical studies have reported altered tryptophan, dopamine, GABA and vitamin B6 metabolism in ADHD, which suggests that microbial metabolism may be affected, but studies directly addressing this question are needed (11).
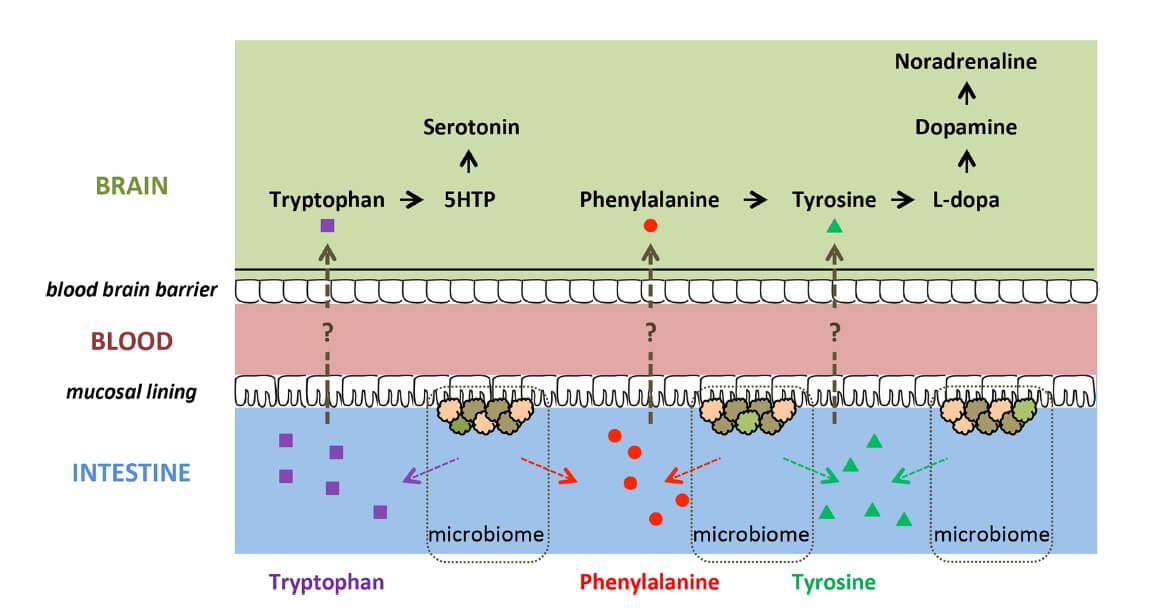
Preclinical evidence indicates that several members of the gut microbiota produce precursors of monoamines ![]() involved in ADHD (i.e. dopamine, tryptophan, noradrenaline, serotonin and GABA) (24,25,26). These precursors (i.e. phenylalanine, tyrosine, tryptophan) might be absorbed through the intestinal epithelium, entering the portal circulation (23) and crossing the blood-brain barrier, potentially influencing host monoamine synthesis (Figure 2). Therefore, differences in abundance and/or metabolic activity of monoamine precursor-producing microbiome bacteria may affect monoamine-related brain functioning and behaviours relevant to ADHD (15).
involved in ADHD (i.e. dopamine, tryptophan, noradrenaline, serotonin and GABA) (24,25,26). These precursors (i.e. phenylalanine, tyrosine, tryptophan) might be absorbed through the intestinal epithelium, entering the portal circulation (23) and crossing the blood-brain barrier, potentially influencing host monoamine synthesis (Figure 2). Therefore, differences in abundance and/or metabolic activity of monoamine precursor-producing microbiome bacteria may affect monoamine-related brain functioning and behaviours relevant to ADHD (15).
Figure 2. Potential routes in which precursors of monoamines ![]() could influence brain functioning (15) CC BY 4.0
could influence brain functioning (15) CC BY 4.0
Immune dysregulation and inflammation
ADHD is linked to altered metabolic and immunologic function, indirectly suggesting that major functions of the gut microbiome may be affected (11).
Immune dysregulation in autoimmune disorders and ADHD may be associated with an altered microbiome, low-grade inflammation, and gastrointestinal dysfunction (27,28,29,30). Dysbiosis may result in a disproportionate quantity of pro-inflammatory microbes leading to increased intestinal permeability and inflammation, as well as a resultant shift of microbes into the systemic circulation which may lead to low-grade systemic inflammation and immune dysregulation (3).
Allergic disease (e.g. atopic eczema) is associated with an increased risk of ADHD (31–33). Conversely, alteration in the microbial colonisation during early life has been suggested to play an important role in susceptibility to developing allergies (34).
Gastrointestinal disturbance in ADHD
A small but growing body of evidence describes an increased incidence of gastrointestinal symptoms (constipation and flatulence) in individuals with ADHD which may be suggestive of an altered microbiome (3).
Dietary Effects on ADHD
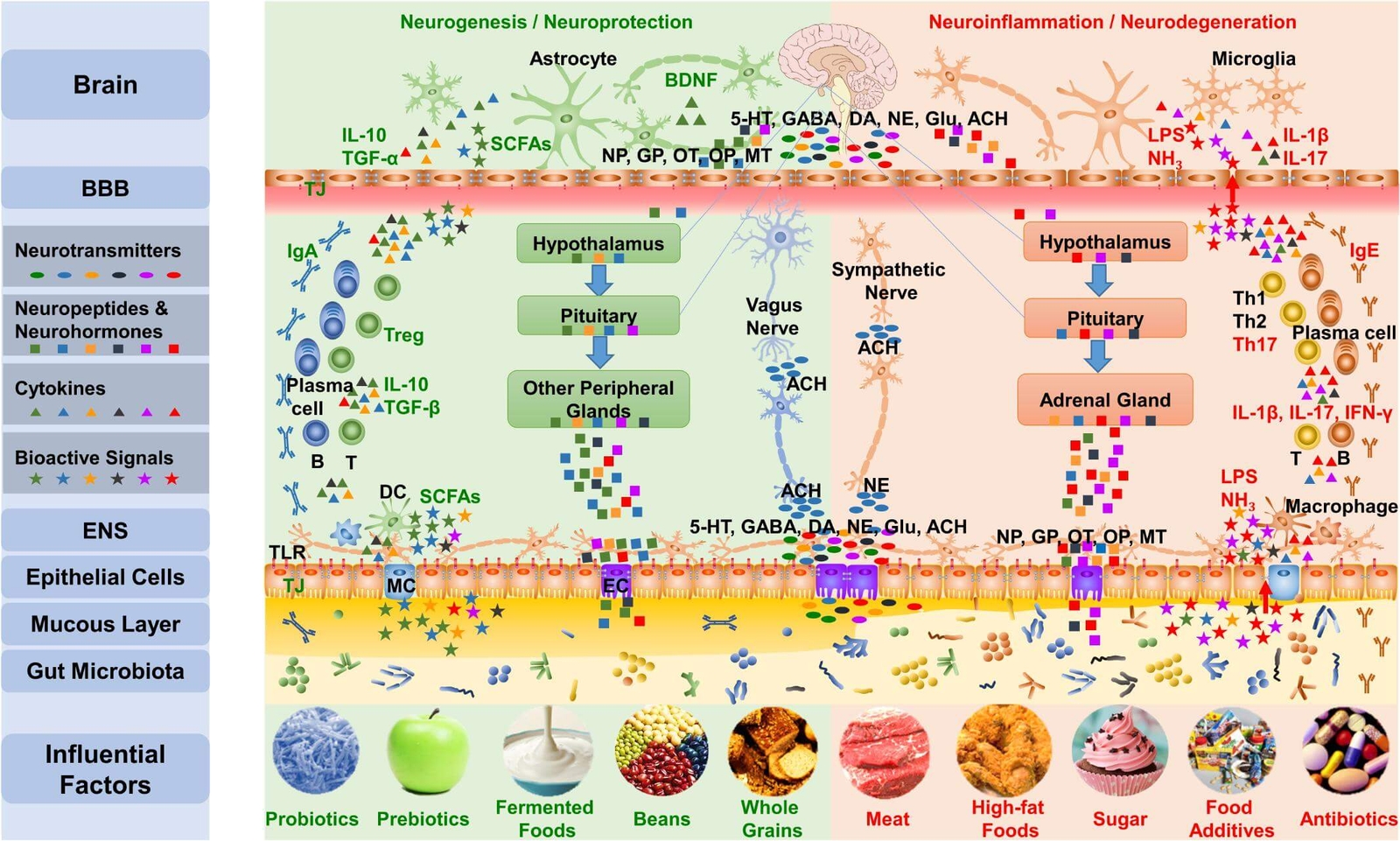
It is well known that dietary factors are associated with childhood behavioural disorders such as ADHD (35). Diet might influence behaviour and ADHD symptoms by affecting the gut microbiome (36,37). Meta-analyses show that the elimination of potential allergens through restriction diets in ADHD may lead to a significant reduction in symptoms, although there is a large degree of variety across studies (38,39,40). Figure 3 highlights influential dietary factors affecting the gut microbiota and their effect on the brain which might conceivably influence the development of ADHD.
Figure 3. Dietary factors and their effect on gut-brain communication (12) CC BY 4.0
Aetiological factors of ADHD
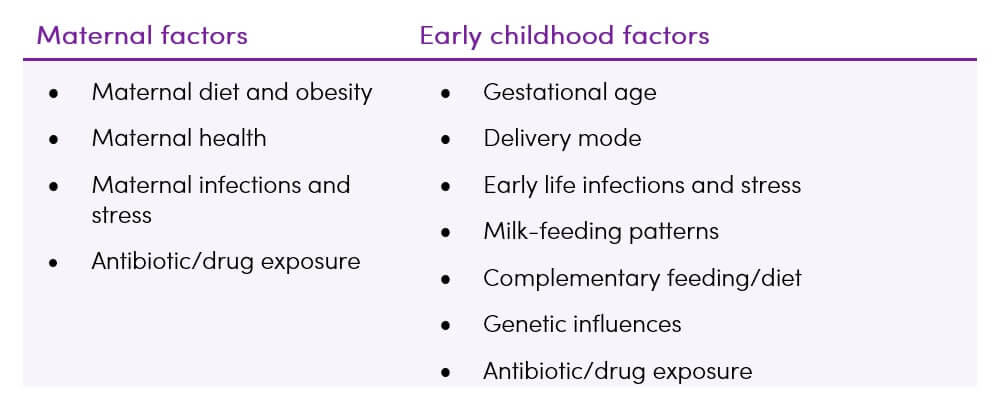
The risk of developing ADHD has been suggested to be associated with many perinatal ![]() risk factors which are able to shape the gut microbiota composition. Maternal factors affect in-utero brain development while early childhood factors affect brain function and behaviour (10,37).
risk factors which are able to shape the gut microbiota composition. Maternal factors affect in-utero brain development while early childhood factors affect brain function and behaviour (10,37).
Table 1. Environmental factors influencing the development of infant gut microbiota (10)
Takeaway on ADHD and the Microbiome
- The gut-brain axis may be a potential intervention target for treating developmental disorders including ADHD (10)
- Initial clinical results indicate an altered composition of gut microbiota in ADHD in early life, but more research is needed (11)
- Preliminary human studies suggest that dietary components modulating gut microbiota may influence ADHD development or symptoms (10)
- If dysbiosis/decreased biodiversity of the microbiome and an unfavourable microbiota development in early life is a controlling mechanism driving ADHD development and symptom severity, any intervention reducing inflammation can be predicted to reduce symptoms (11)