Evidence is accumulating on the potential clinical benefits of prebiotics, probiotics and synbiotics in treating chronic liver diseases including non-alcoholic fatty liver disease (NAFLD/NASH), alcohol-related liver disease (ALD) and liver cirrhosis (1). Beneficial effects vary widely in individuals due to vast differences in diet, genetic background and gut microbiome composition and there is limited consensus available in the evidence for specific strains/doses for treatment options in gut-liver axis dysfunction (2). However, the underlying mechanisms of gut dysbiosis and gut permeability highlight the importance of ensuring a healthy and diverse microbiome as the goal of any intervention.
Treatment Options
Based on an individual’s microbiota composition, personalised treatment would identify the most promising microbiome-altering treatment plan (3).
Treatment options in non-alcoholic fatty liver disease (NAFLD/NASH)
Probiotics, prebiotics and synbiotics have been shown to play pivotal roles in the treatment and prevention of NAFLD. However, inconsistent experimental results need rigorous experimental designs investigating type/strain and effective doses in order to verify the therapeutic effects (2,4,5,6).
Probiotics
Several meta-analyses show that probiotics significantly reduce the development of NAFLD in adults and children. In particular, the use of probiotics is associated with lower plasma aminotransferase and total cholesterol levels, lower systemic inflammation and improved insulin resistance. Using different regimens for 8 to 30 weeks can produce positive effects (7,8,9,10).
Lactobacillus and Bifidobacterium are frequently used for their beneficial effects on NAFLD. Probiotic therapies may ameliorate insulin resistance by reducing serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), total cholesterol and TNF-α in NAFLD patients (11).
Some of the research highlighting the effectiveness of probiotics in the management of NAFLD/NASH is given below.
- VSL#3 supplementation (2 sachets twice per day) improved functional liver markers in NAFLD and alcoholic liver cirrhosis (12). VSL#3 is a mixture containing 450 billion bacteria in various strains (Streptococcus thermophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus bulgaricus)
- Treatment with bulgaris or S. thermophilus reduced functional liver markers (13)
- Supplementation with rhamnosus GG resulted in a significant improvement in liver function in obese children with NAFLD (14)
- Treatment for 4 months with VSL#3 resulted in a significant improvement in the severity of fatty liver and a significant decrease in body mass index of children with NAFLD (15)
- 12 weeks of supplementation with a multi-strain probiotic (containing Lactobacillus acidophilus ATCC, B3208; Bifidobacterium lactis DSMZ 32269; Bifidobacterium bifidum ATCC SD6576; Lactobacillus rhamnosus DSMZ 21690) reduced the levels of ALT, AST, cholesterol, low-density lipoprotein-C (LDL-C), triglycerides and waist circumference in children with NAFLD (16)
- Consuming yoghurt containing acidophilus and B. lactis for 8 weeks in adult patients with NAFLD was associated with a significant improvement of transaminases and cholesterolaemia (17)
- Improvement in cytokine profile, insulin action and hence glycaemic control was demonstrated in NAFLD after 8 weeks of supplementation with a multi-strain probiotic (Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum and Streptococcus thermophiles) (18)
Prebiotics and Synbiotics
Synbiotic supplementation has a favourable effect on inflammatory factors, liver enzymes and some anthropometric indices ![]() , lipid profiles and glucose homeostasis parameters in individuals with NAFLD (19). A systematic review indicated that synbiotic supplements that contained 20 million - 5 billion CFU bacterial strains and 125mg - 10g prebiotic for 8 - 28 weeks is suggested for NAFLD patients (19).
, lipid profiles and glucose homeostasis parameters in individuals with NAFLD (19). A systematic review indicated that synbiotic supplements that contained 20 million - 5 billion CFU bacterial strains and 125mg - 10g prebiotic for 8 - 28 weeks is suggested for NAFLD patients (19).
- Inulin-type fructans such as oligofructose (16g/day) over 8 weeks improved markers of liver function and disease and decreased insulin levels in individuals with NASH (20)
- A combination of Bifidobacterium longum and fructooligosaccharide (FOS) administered over 24 weeks reduced TNF-α, CRP, aspartate aminotransferase (AST), insulin resistance and lipopolysaccharide (LPS) as well as decreased NASH activity index in individuals with NASH (21)
- 24-week consumption of synbiotic yoghurt (containing 108 CFU Bifidobacterium animalis/mL and 1.5 g inulin, 300g daily) improved hepatic steatosis and liver enzyme concentrations in individuals with NAFLD (22)
- Synbiotic supplementation, twice daily over 28 weeks (200 million bacteria of seven strains: Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum and Lactobacillus bulgaricus) with prebiotic (125mg FOS)) improved the main features of NAFLD in NAFLD patients with normal or low BMI (23)
Diet
Although diet can significantly alter both the composition and function of the gut microbiota, clinical studies that link dietary interventions with gut microbiota changes for the treatment of NAFLD/NASH are limited (24).
- A high-energy, high-fat, high-carbohydrate diet and decreased choline intake alters the gut microbiota and is associated with NASH. Correcting dietary habits is typically part of the standard recommendations for NASH treatment (25)
- A 3-week high carbohydrate low-fat diet with inulin (prebiotic) reduced hepatic lipogenesis and serum triglyceride levels in a small study (26)
- A fibre-rich diet is associated with greater richness and diversity of the gut microbiota and is positively associated with the presence of Bacteroidetes and Actinobacteria and a reduction in the Firmicutes:Bacteroides ratio (27). Fibre-rich diets also promote a higher concentration of SCFA, especially butyrate, which has a beneficial effect on inflammation. The replacement of fructose, and other simple carbohydrates, with non-digestible carbohydrates, can avoid the consequences of dysbiosis in the gut-liver axis, especially in NASH (25)
- Excess protein has been linked with potentially damaging effects on the gut microbiota and health (28). A high-protein diet results in reductions in butyrate-producing bacteria which may increase the risk of NASH (25). Generally, high protein intake is accompanied by a reduction in carbohydrate intake. Therefore, it is possible that the impact of the consumption of high-protein diets on the gut microbiota is related not only to the production of toxic substances derived from protein fermentation but also to the reduction of dietary carbohydrate consumption, especially of non-digestible carbohydrates
Table 1. Summary of clinical trials of gut microbiota-targeted therapies on NAFLD/NASH. Adapted from (29) CC BY 4.0
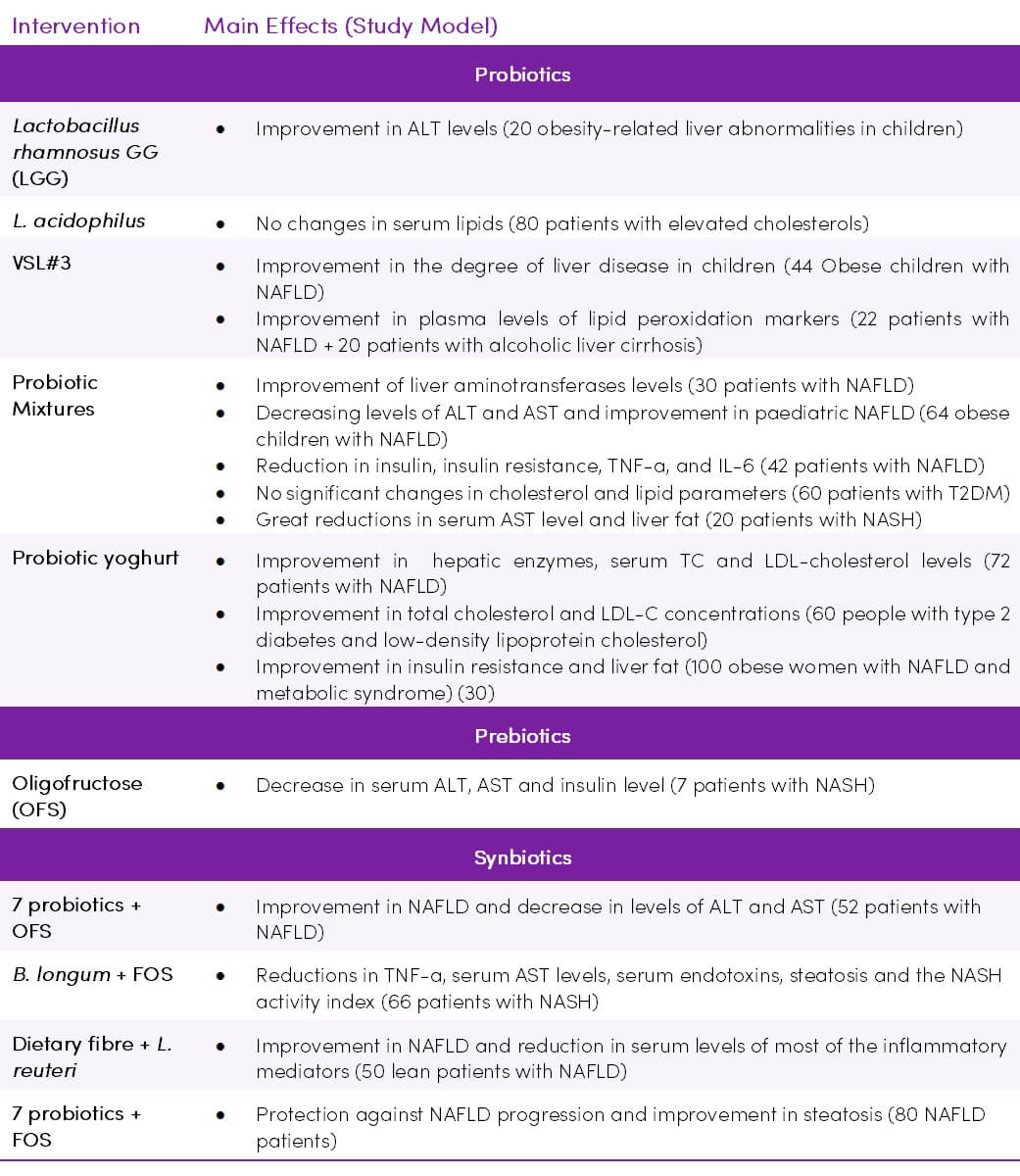
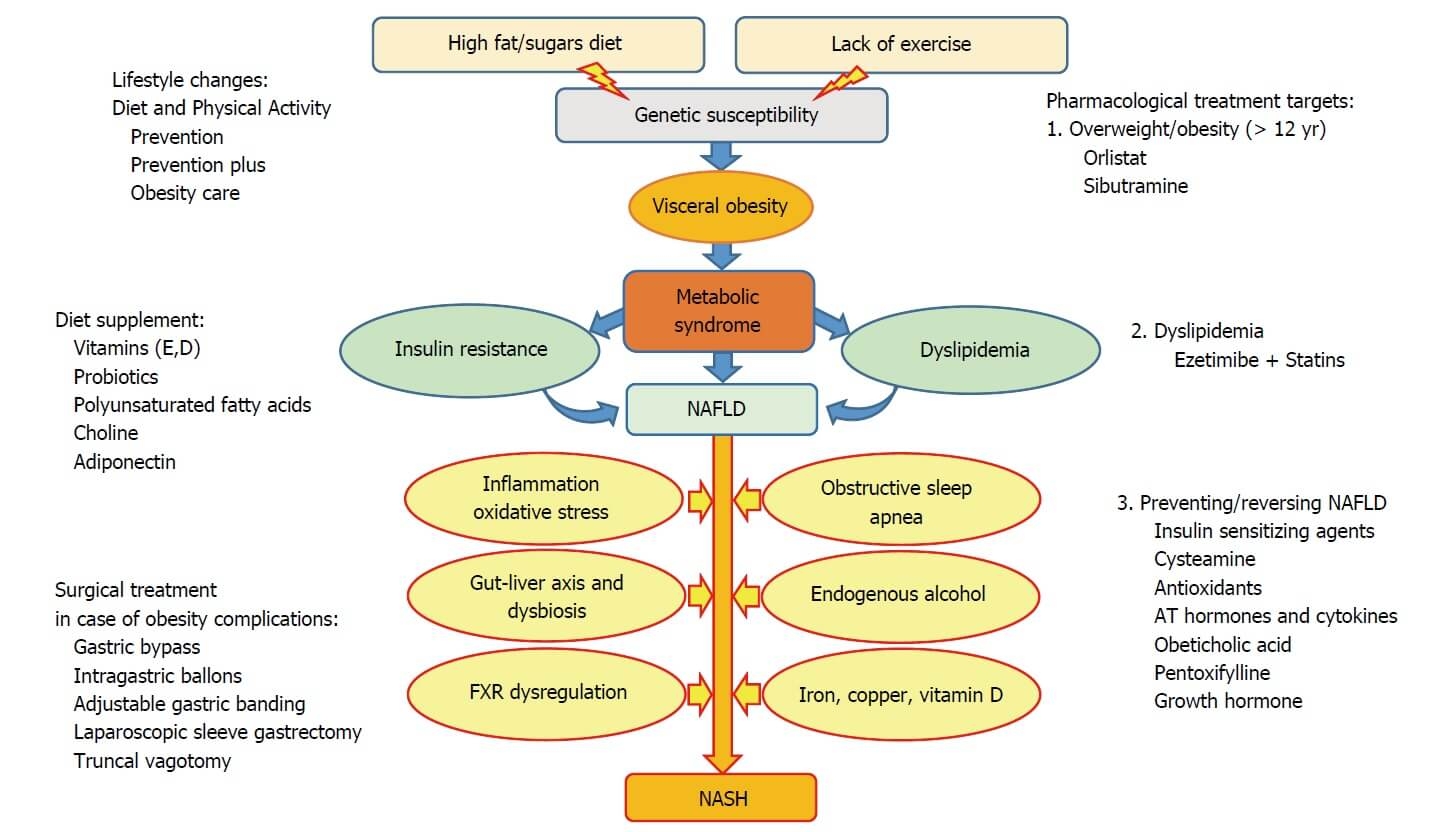
Figure 1. Multiple pathomechanisms involved in development and progression of non-alcoholic fatty liver disease, and proposed stepwise treatment options (46) CC BY-NC 4.0
Treatment options in alcohol-related liver disease (ALD)
Recent evidence suggests that probiotics have the ability to transform the composition of the intestinal microbiota community, which leads to the reduction of alcohol-induced dysbiosis, intestinal permeability, bacterial translocation, endotoxaemia, and the development of ALD (2,31). While the transformation of intestinal microbiota by probiotics appears to be a promising therapeutic strategy for the treatment of intestinal barrier dysfunction, there is a scarcity of research that studies probiotics in the context of ALD (31).
- Administration of casei Shirota (6.5 × 109 CFU, three times daily for 4 weeks, with alcohol abstinence) can restore neutrophil phagocytic capacity in alcoholic cirrhosis, possibly by altering IL-10 secretion and toll-like receptor TLR-4
 expression (32)
expression (32) - Yakult 400, containing casei (with abstinence state) significantly increased serum levels of liver-specific rapid-turnover protein transthyretin and decreased the level of inflammatory markers in liver cirrhosis patients. In addition, alcohol-induced deterioration of gut flora was improved with the increase of protein production induced by probiotic treatment (33)
- Supplementation with bifidum and L. plantarum 8PA3 for 5 days may restore the bowel flora and improve alcohol-induced liver injury when combined with standard therapy in ALD patients (34)
Treatment options in portal hypertension
Modulation of the gut microbiota via the administration of probiotics has shown promising results in haemodynamic changes in portal hypertension (24).
Six weeks of oral VSL#3 probiotic (2 sachets per day) improved the hepatic and systemic haemodynamics in individuals with cirrhosis (35). Similarly, adjunctive VSL#3 use has been shown to improve the response rate to propranolol ![]() therapy and is safe and well tolerated in individuals with cirrhosis (36).
therapy and is safe and well tolerated in individuals with cirrhosis (36).
Treament options in liver transplantation
A meta-analysis of 4 controlled studies demonstrates that giving synbiotics one day before or on the day of transplantation reduces the rate of infections after surgery and shortens hospital and ICU stay as well as antibiotic usage (37). Pre liver transplant administration (for a period of 1 – 10 weeks) of a 4-strain probiotic (Lactococcus lactis PB411, Lactobacillus casei PB121, Lactobacillus acidophilus PB111 and Bifidobacterium bifidum PB211) can help to prevent post-operative infections (38).
Mechanisms of action
The role of the gut microbiome in liver disease is underscored by gut dysbiosis and gut permeability.
Figure 2. Mechanisms by which gut bacteria affect the hallmarks of nonalcoholic fatty liver disease (39) CC BY 4.0
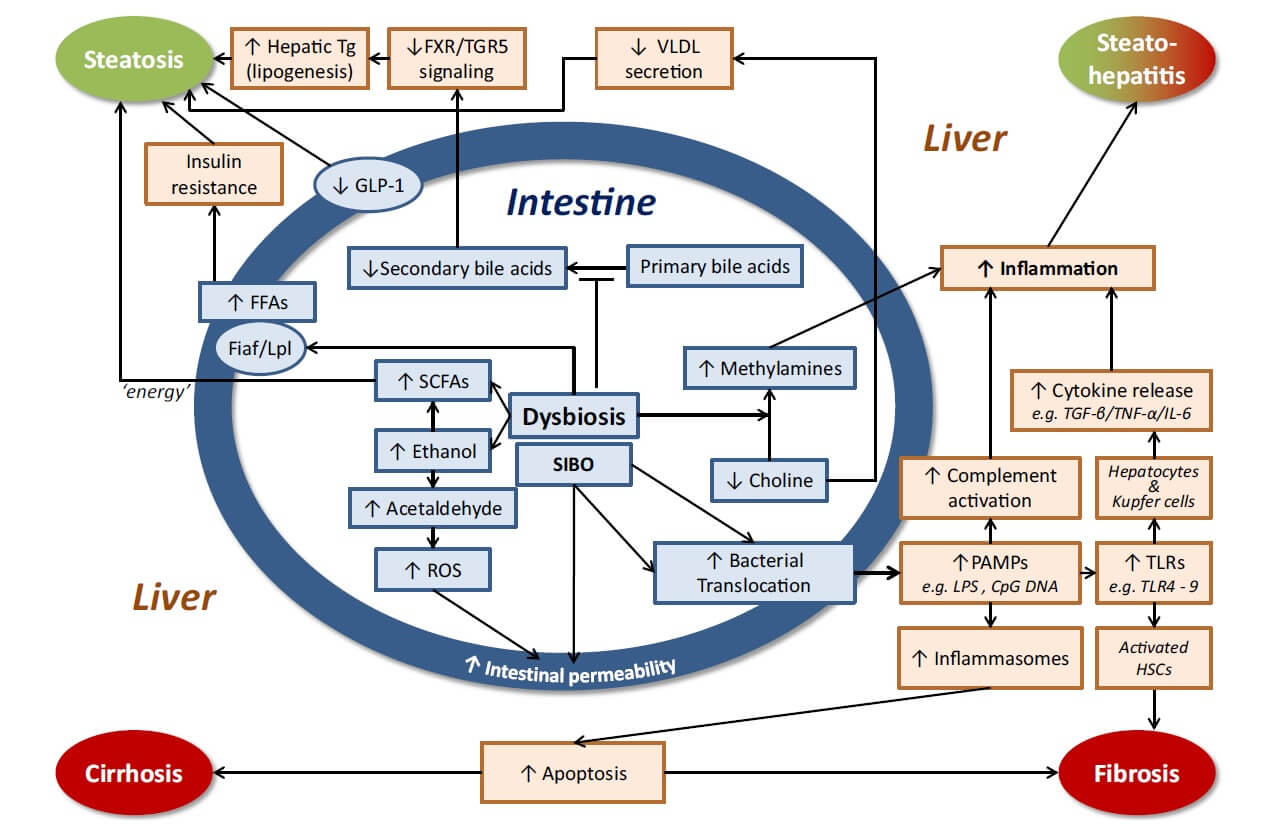
Table 2. Microbiota related mechanisms of action in NAFLD/NASH (4,11,25,40,41,42).
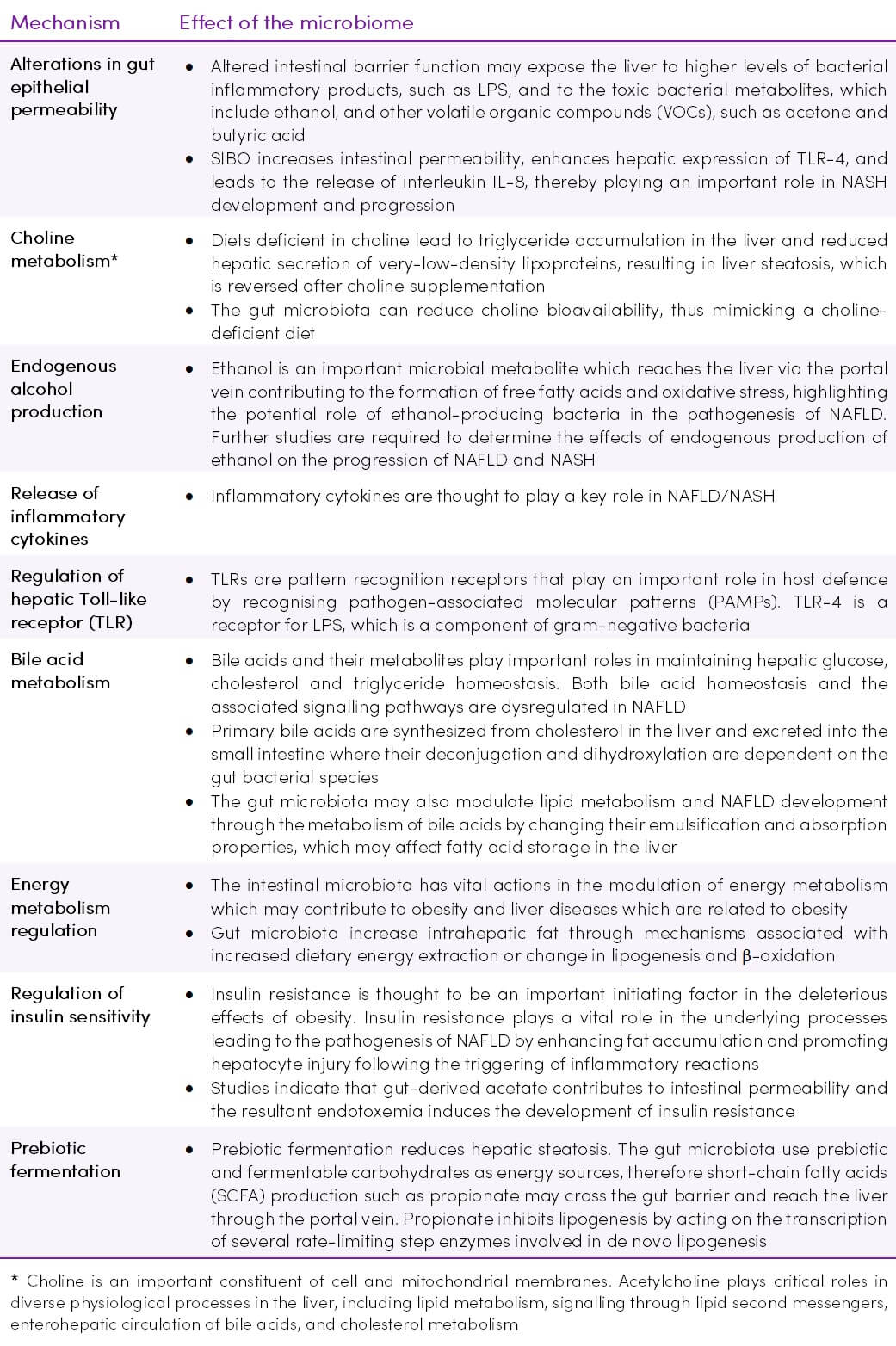
Figure 3. Gut-liver axis components in normal conditions (right part) and in non-alcoholic fatty liver disease (NAFLD) (47) CC BY 4.0
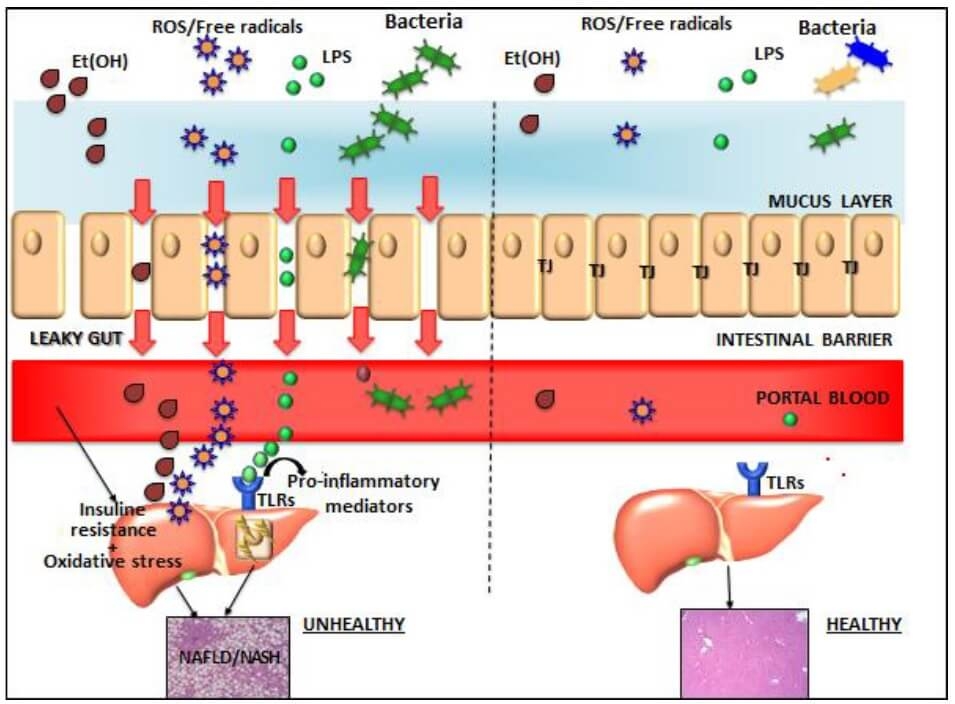
Table 3. Mechanism of action - other liver conditions (11,24,31,41,43,44,45)
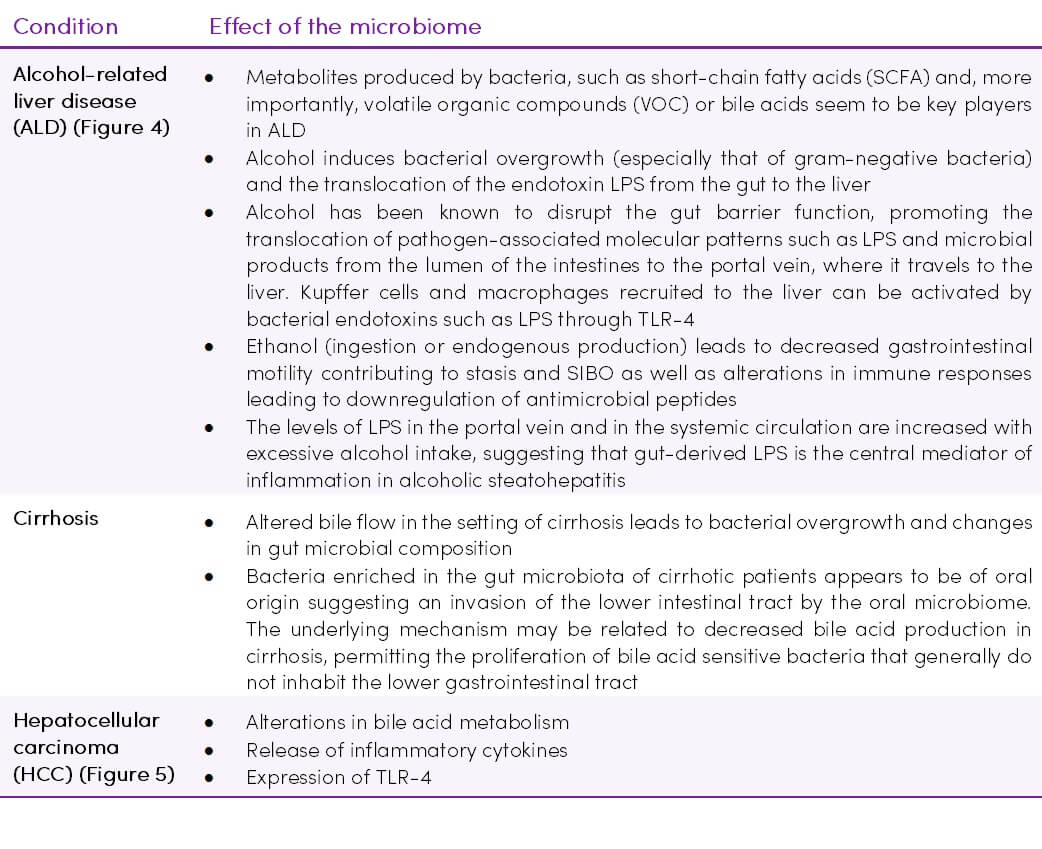
Figure 4. The effects of chronic alcohol ingestion on the intestinal microbiome during the development of alcoholic liver disease. Adapted from (49) CC BY-NC 3.0
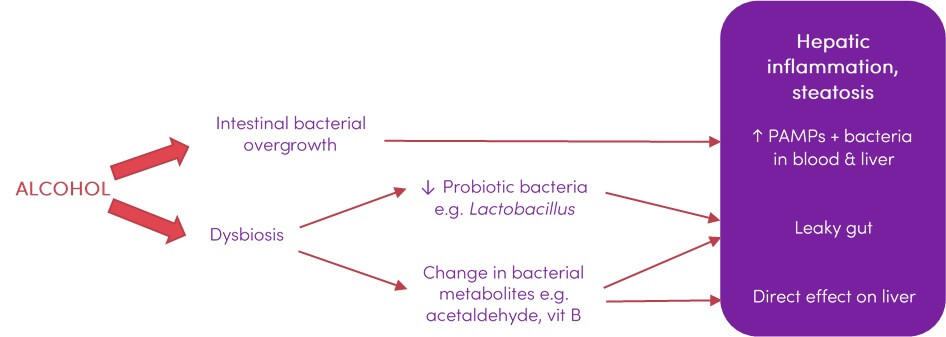
Figure 5. Effects of the intestinal microbiota on HCC tumourigenesis (11) CC BY-NC-ND 4.0
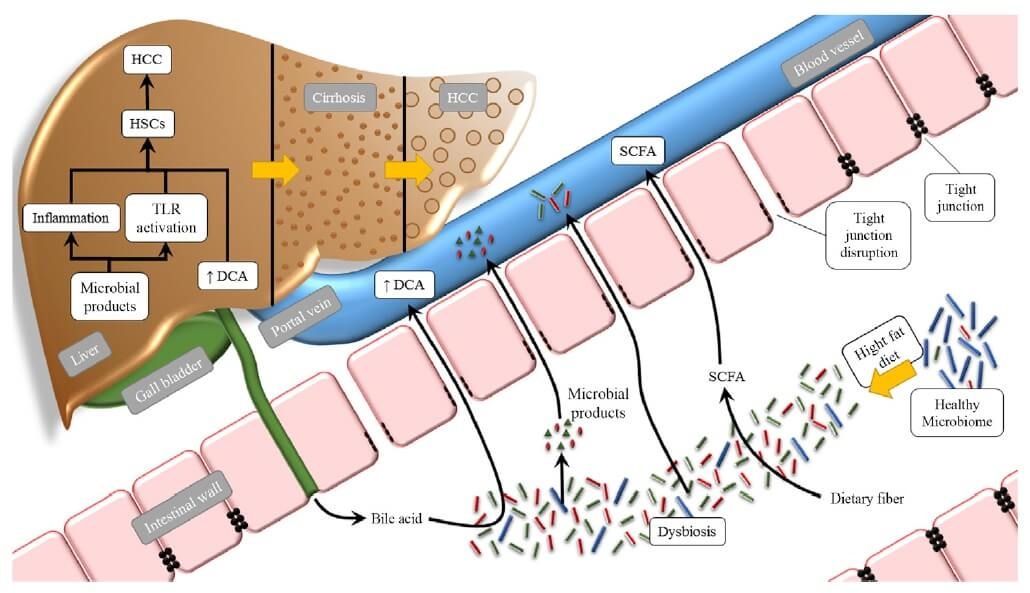
Abbreviations: HCC, hepatocellular carcinoma; HFD, high-fat diet; TLR, Toll-like receptor; HSCs, hepatic stellate cells; DCA, deoxycholic acid; SCFA, short-chain fatty acid.
Takeaway on the gut-liver axis
- Studies suggest that microbial factors are driving forces in many different liver diseases and at various stages of liver diseases. The microbiota affects diverse pathophysiological processes such as hepatic steatosis, liver inflammation, fibrosis development and hepatic encephalopathy. Studies have now convincingly established that the microbiota plays a critical role in these diseases (42)
- Many studies strongly suggest that targeting the gut microbiome through the use of probiotics, prebiotics, synbiotics and possibly faecal microbiota transplantation (FMT), may be an effective treatment for NAFLD and ALD, improving several parameters including insulin resistance, plasma levels of transaminases and degree of lipid infiltration of the liver (41)
- Large-scale prospective studies are required to determine how the gut microbes and their metabolites are altered in various clinical settings of liver disease, and to determine which microbial strains affect disease phenotype, that is, either acting protective or detrimental (8,11,41,42)
- There is still no strong evidence indicating which comes first in ALD; increased permeability and then dysbiosis or dysbiosis inducing increased permeability. It is also not known exactly how direct alcohol toxicity contributes to these changes. ALD can be developed without gut microbiota change and all ALD patients do not have same microbiota composition, therefore, further clinical trials are needed to elaborate on new therapeutic options and mechanisms (31)