Anorexia nervosa (AN) is a complex and debilitating psychiatric disorder defined by extremely low body weight, a fear of weight gain, and image disturbance (1). The lifetime prevalence of AN for females ranges from 0.3%-1.5% and 0.1%-0.5% for males (2). It has the highest mortality rate of any psychiatric illness, and only one-half of patients experience long-term recovery. In addition, patients with AN often experience psychological co-morbidities, including anxiety (72% lifetime prevalence) (3) and depression (more than 81% lifetime prevalence) (4), further complicating treatment.
The causes and subsequent development of AN are complex and poorly understood. Genetic, psychological, sociocultural, and biological factors contribute to the risk of AN onset and maintenance (5,6). Nutritional rehabilitation procedures and psychotherapy are the mainstay treatments for AN (7,8); however, considering the high mortality and high chronicity, there is an urgent need to explore potential adjunct therapies.
New research is shedding light on the role of the gut microbiota in AN. Compared to healthy individuals, the AN microbiome demonstrates significant differences in composition (dysbiosis), represented by low microbial diversity and taxonomic differences (9,10,11,12,13). The gut microbiome could play an important role in the disorder by regulating key features of AN, including appetite control, energy metabolism, gastrointestinal physiology, and mood. The microbiota may regulate these aspects through various immune, neuroendocrine, and metabolic pathways, forming the microbiota-gut-brain axis (14). While the relationship between the microbiome, dietary patterns and AN disease onset and progression remains poorly defined, preliminary studies suggest targeted microbiome-based interventions, including pre and probiotics and faecal microbiota transplants (FMT), may be beneficial adjuncts to standard AN therapy (14,15).
The gut microbiome and dysbiosis in AN
Microbial diversity is essential for health and disease prevention. A diverse microbiome includes species from the phyla Actinobacteria, Verrucomicrobia, Proteobacteria, Firmicutes, and Bacteroidetes (16). The gut microbiota of healthy individuals is far more diverse than the microbiota of AN individuals. However, there are conflicting results regarding specific changes in the microbiome in patients with AN, which could be due to patient dietary habits, lifestyle (e.g. stress, medication use and exercise levels) and the severity and duration of disease (15,17).
The majority of studies found lower alpha microbial diversity ![]() in AN patients compared to healthy controls (9,10,11,12,13); however, three studies found no difference in alpha diversity (18,19,20). In addition, two clinical trials following gut microbiome composition before and after nutritional rehabilitation found that weight gain did not restore bacterial diversity to levels comparable to normal-weight controls (9,19). These results suggest that dysbiosis might persist beyond weight recovery and potentially contribute to relapse, but further research is required.
in AN patients compared to healthy controls (9,10,11,12,13); however, three studies found no difference in alpha diversity (18,19,20). In addition, two clinical trials following gut microbiome composition before and after nutritional rehabilitation found that weight gain did not restore bacterial diversity to levels comparable to normal-weight controls (9,19). These results suggest that dysbiosis might persist beyond weight recovery and potentially contribute to relapse, but further research is required.
In general, a reduction in microbial diversity is associated with impaired immune defence and reduced capacity for harvesting calories from the diet (21), which may be relevant for AN pathophysiology. Furthermore, clinical symptoms of AN, including eating disorder psychopathology, anxiety symptoms and depression were related to a change in the composition and reduction in the diversity of gut microbiota, especially butyrate-producing bacterial species (9,20).
Current research demonstrates that acutely ill AN patients show a relative depletion of several carbohydrate-fermenting taxa belonging to the Firmicutes phylum (e.g. Roseburia, Clostridium, Anaerostipes, and Faecalibacterium prausnitzii), while Bacteroidetes (9,11,12,19,20) and Proteobacteria (Escherichia coli) are enriched (20,22). In contrast, the gut microbiome of individuals who are obese has an increased level of Firmicutes, and a reduced level of Bacteroidetes (23).
Increased abundance in the methane-producing archaeon Methanobrevibacter smithii (9,20,22,24) is consistently reported in AN studies. M. Smithii produces methane by metabolising excess hydrogen and carbon dioxide in the gut. It also enhances nutrient transformation into calories and increases fermentation of fibre and resistant starch, generating short-chain fatty acids (24). Therefore, the increase in the methanogens such as M. Smithii may be an adaptive response to prolonged caloric restriction in AN.
Studies suggest decreased levels of carbohydrate-fermenters in AN individuals are accompanied by a shift toward mucin-degrading microorganisms (e.g. Verrucomicrobia, Bifidobacteria). These microorganisms favour delayed colonic transit commonly occurring in AN (25), and especially in a nutrient-deficient environment, they survive by digesting the protective intestinal mucus layer. Specifically, the mucin-degrader Akkermansia muciniphila found in several AN studies may represent an adaptive response (19,26). A. municiphilia is a symbiotic bacterium residing in the intestinal mucus layer and utilises intestinal mucin as its sole carbon, nitrogen, and energy source, especially in the absence of other dietary sources (27,28). A. municiphilia levels are positively associated with weight loss and are inversely associated with inflammation, body weight, obesity, and metabolic disorders (29,30).
Microbial metabolites in AN
The gut microbiota interacts with intestinal cells, the enteric nervous system, and the central nervous system, forming the microbiota-gut-brain axis. The microbiota produces various metabolites, including short-chain fatty acids (SCFAs), tryptophan metabolites, bile acids, neurotransmitters, and hormones. These bacterial metabolites can act on the intestinal epithelial barrier, cross the blood-brain barrier, and act directly on brain neurons, thereby affecting neurogenesis, cognitive function, and mood (31). Furthermore, microbial metabolites have immunomodulatory effects and can influence host appetite and eating behaviour by directly affecting nutrient sensing, appetite, and satiety-regulating systems (32).
Increased levels of faecal SCFAs are observed in obese and overweight people (33,34). However, faecal metabolite levels in AN patients show reduced SCFAs, particularly butyrate (19,20,35,36), compared to healthy subjects. Additionally, several butyrate-producing taxa belonging to the Firmicutes phylum (Roseburia, Clostridium, Eubacterium) are consistently underrepresented in AN gut microbiota (9,11,19,20).
Associations have been found between bacterial species, stool SCFA levels and clinical presentation in AN patients. In particular, the relative abundance of Roseburia spp. was correlated with decreased faecal butyrate levels (19,20), which were associated with increased anxiety and depression scores in AN patients (20).
Bacterial species produce several neuroactive compounds that may affect the peripheral and central nervous systems and human behaviour. For example, serotonin is one of the primary neurotransmitters present in the brain; however, more than 90% is produced in the gut, and its production is influenced by diet. Significantly lower brainstem serotonin levels have been observed in anorectic mice, which may be associated with reduced tryptophan intake resulting from restricted food intake (37). Recently, lower levels of serotonin, dopamine and gamma-aminobutyric acid (GABA) were detected in faecal samples of women with AN when compared with healthy women (38). In this study, an increased abundance of of Alistipes was observed. Alistipes is a genus of bacteria that can hydrolyse tryptophan (serotonin precursor) to indole and thus decrease serotonin availability (39).
Intestinal permeability and inflammation in AN
The gut mucosal barrier acts as a buffer between the external environment and the host's physiology and is essential to proper immune system development and function. A compromised gut epithelium and the subsequent bacterial translocation may trigger immune and inflammatory responses which can promote neuroinflammation across the blood-brain-barrier, and impact numerous functions that are dysregulated in AN including mood and feeding behaviour (40,41).
Chronic food restriction in AN may induce gut barrier dysfunction due to an increase in mucin degrading microorganisms (19,26), and altered tight junction protein expression which reduces gastric wall thickness (42). Furthermore, decreased availability of colonic butyrate may also lead to an inflammation-mediated disruption of the intestinal barrier in AN patients (43).
In two recent meta-analyses, patients with AN showed a low-grade inflammatory state with increased TNF-α, IL- 6, and IL-1β (44,45). Whether this systemic inflammation results in neuroinflammatory responses is largely unexplored in AN patients, although animal AN models suggest a role of microglia and neuroinflammation in AN (46,47,48).
Autoimmunity in AN
Emerging evidence suggests that autoantibodies due to increased gut permeability could play a role in AN pathophysiology. Specifically, an increased abundance of autoantibodies against appetite modulating neuropeptides, such as α-melanocyte-stimulating hormone (α-MSH), have been identified in AN (49,50). In addition to central feeding regulation, α‐MSH signalling is involved in anxiety and depression (51).
Subsequent studies found that Escherichia coli, which are overrepresented in the gut of AN patients (11), produce caseinolytic protease B (ClpB), a heat-shock protein that can act as a partial homologue to α- MSH and cross-react with receptors of appetite- and satiety-regulating hormones. ClpB protein concentrations correlate positively with α-MSH-reactive IgG for all patients with eating disorders, thus may play an important role in AN development (52).
The link between AN, autoimmune disease and the gut microbiota is an area that warrants further research. Studies to date have found a strong bidirectional relationship between autoimmune disease and eating disorders, including AN (53). Specifically, gastrointestinal-related autoimmune diseases such as coeliac disease and Crohn's disease show a bidirectional relationship with AN, and the previous occurrence of type 1 diabetes increases the risk for AN (53).
Microbiota-gut-brain axis in AN
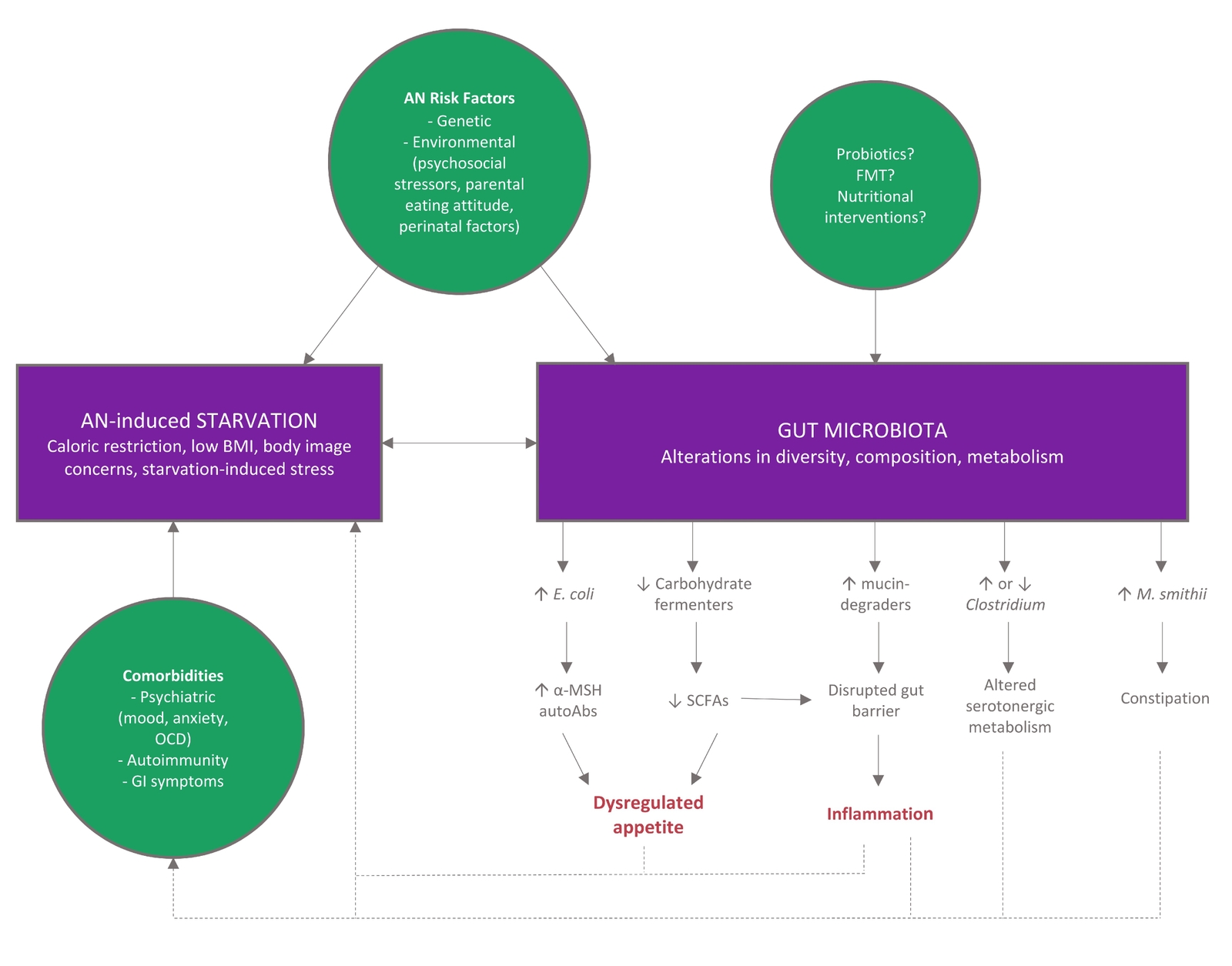
To date, research cannot identify if altered gut microbiota is a cause or consequence of the long term reduced food consumption that underpins AN. However, regardless of which comes first, alterations in the microbiota-gut-brain axis are likely to contribute to disease chronicity and relapse, with altered immune system function, poor SCFA and butyrate production, and increased intestinal permeability perpetuating the cycle (Figure 1).
Figure 1 Model for gut microbiome involvement in AN pathophysiology. Adapted from (15) CC BY 4.0
Implications for treatment
Weight restoration and renourishment in AN typically involves high calorie, high fat/protein diets, which can cause gastrointestinal discomfort and may cause further dysbiosis and inflammation (54). Few studies have investigated the microbiome after nutritional rehabilitation in AN patients; however, initial results suggest bacterial diversity is not restored, and elevated levels of mucin and protein-degrading taxa, and reduced levels of butyrate-producing bacteria may persist (19). Therefore, including fibre in nutritional rehabilitation programs is an important consideration for restoring healthy gut microbiota composition and improving health outcomes in AN patients.
Similarly, it is vital to consider the type of dietary fat incorporated into rehabilitation programs. Omega-3 polyunsaturated fatty acids (n-3 PUFAs) can modulate the diversity and abundance of gut microbiota (55) and exert important anti-inflammatory and immunomodulatory effects, which are relevant to AN. For example, n-3 PUFAS can increase the abundance of butyrate-producing bacteria and decrease levels of pro-inflammatory bacteria; and improve gastrointestinal homeostasis (56). Furthermore, n-3 PUFA supplementation may increase appetite (57), help weight gain (58), maintain integrity and restore disturbed blood-brain-barrier function, and stabilise neuronal membrane fluidity index (59), thereby positively affecting cognition and mood. Several studies suggest that the clinical benefits of n-3 PUFA treatments in AN may include weight regulation and mood improvement, but further studies are required (60,61,62).
The administration of pre and probiotics provides potential adjunctive treatment options for neuropsychiatric conditions (63), cognitive impairment (64), and gastrointestinal dysfunction (65), all relevant to AN. Furthermore, several clinical trials indicate clinical symptoms of AN are related to levels of SCFAs and butyrate-producing bacteria; thus, clinical trials investigating the effects of pre and probiotics in AN are warranted.
FMT from healthy donors is currently being explored as a potential strategy for re-establishing a diverse microbiota and improving the outcomes of nutritional rehabilitation. Recent case studies of FMT in AN patients show promising results with improvements in weight gain, microbiota composition and gut barrier function (66,67).
Takeaway on the Microbiome and AN
- Compared to healthy individuals, the microbiome of AN patients demonstrates significant differences in diversity and composition, although further studies are required to determine if differences are a cause or consequence of AN.
- Microbial dysbiosis alters the production of microbial metabolites which affect metabolism, appetite, satiety and mood.
- Microbial dysbiosis in AN patients may enhance intestinal permeability leading to neuroinflammatory and autoimmune responses that perpetuate disease pathogenesis.
- Current nutritional rehabilitation therapies do not restore the gut microbiome in AN patients and further studies are required to optimise rehabilitation strategies, potentially incorporating adjunct therapies that target the microbiome.