Introduction
Irritable bowel syndrome (IBS) is a chronic, often debilitating, and highly prevalent spectrum of symptoms arising from dysfunctions of the gut-brain axis (1). Gastrointestinal (GI) symptoms include recurrent abdominal pain, disordered defecation (abnormal stool form, stool frequency, or both) and bloating (2). Non-GI symptoms include back pain, gynaecological and bladder symptoms, headache, and fatigue (3).
Symptoms include abnormal intestinal motility or transit, increased sensation or perception of abdominal symptoms such as pain or bloating (mediated in the gut or the brain), and psychological disturbances, including somatisation ![]() or multiple somatic comorbidities (4).
or multiple somatic comorbidities (4).
IBS is different from inflammatory bowel disease (IBD), which includes chronic health conditions such as Crohn's disease and ulcerative colitis.
IBS is one of the most common GI disorders, accounting for 12% of all primary care visits, and is the most common reason for visiting a gastroenterologist (5). The global prevalence of IBS using the Rome III diagnostic criteria is 9.2%, while prevalence according to the more recent Rome IV criteria is 3.8% (6). IBS-D (diarrhoea prominent IBS) is the most common Rome IV subtype (31.5% of IBS sufferers). Women and individuals younger than 50 years are most commonly affected (7).
The aetiology of IBS is complex and not fully understood. Evidence suggests that the potential mechanisms underlying IBS include genetic factors, abnormal serotonin metabolism, chronic infection, low-grade mucosal inflammation, immune activation, visceral hyperalgesia ![]() , changes in gastrointestinal motility and intestinal permeability, gut microbiota, disorders of bile acid metabolism, gut-brain axis dysfunction, and psychosocial factors (8).
, changes in gastrointestinal motility and intestinal permeability, gut microbiota, disorders of bile acid metabolism, gut-brain axis dysfunction, and psychosocial factors (8).
A biopsychosocial model can explain symptoms of abdominal pain and disordered bowel habit in IBS: A genetic predisposition, in which adverse events in early life, psychological factors, or gastrointestinal infections trigger alterations in the enteric nervous system ![]() . Dietary factors alter GI sensation, motility and secretion leading to GI and non-GI symptoms (8). Accumulation of different mechanisms (e.g. psychological, sensory, and motor) increases the severity of GI and non-GI symptoms and causes impairments in quality of life.
. Dietary factors alter GI sensation, motility and secretion leading to GI and non-GI symptoms (8). Accumulation of different mechanisms (e.g. psychological, sensory, and motor) increases the severity of GI and non-GI symptoms and causes impairments in quality of life.
Currently, there is no cure for IBS. The majority of the treatment involves pharmaceutical drugs, non-drug interventions (exercise and diet), and comprehensive therapy (8,9). Patients and primary healthcare providers are often unsatisfied with available pharmacologic remedies and may seek complementary and alternative medicine (CAM) therapies, which may be beneficial for abdominal pain and overall response in IBS (10,11). There is a continued need for novel evidence-based practices for the optimal management of IBS, regardless of whether treatments are CAM or traditional Western medicine.
Traditional Understanding
The initial Manning criteria for IBS, proposed in 1978, used a 15-symptom questionnaire identifying key indicators of IBS: loose stools, relief of pain after a bowel movement, abdominal distension, frequent bowel movements, mucus, and sensation of incomplete evacuation (12). Each of these described symptoms is an important definer of IBS but do not separate IBS based on predominant stool type.
Diagnosis
IBS is diagnosed clinically based on the Rome diagnostic criteria, physical examination and limited diagnostic tests. The Rome IV criteria of 2016, derived by consensus from a multinational group of experts in the field of gut-brain interaction disorders, is the latest diagnostic criteria (Table 1) (1).
Table 1 Rome IV diagnostic criteria (13)
|
Rome IV diagnostic criteria for IBS |
|
Recurrent abdominal pain on average at least 1 day per week in the last 3 months, associated with 2 or more of the following criteria: · Relief or worsening of pain related to defecation · Association with a change in stool frequency · Associated with a change in stool form (appearance) |
|
These criteria should be fulfilled for the last 3 months with symptom onset at least 6 months before diagnosis. |
Although bloating is a commonly reported symptom, its presence is not mandatory to accurately diagnose IBS (1).
The wide variety of symptoms and manifestations make the definite diagnosis of IBS a continued challenge. The differential diagnosis is so extensive that it may take years to establish the correct diagnosis. Exhaustive investigation to exclude all organic pathology is still made in many IBS patients (14).
Biomarkers implemented for IBS diagnosis and management include blood (serum), faecal, immunological, microbiome analysis, microRNAs, and some promising novel biomarkers associated with imaging and psychological features of the disease (14).
Diagnostic Criteria for IBS Subtypes
Predominant bowel habits are based on stool form on days with at least 1 abnormal bowel movement (4).
- IBS with constipation (IBS-C): >25% of bowel movements with Bristol Stool Form Scale (BSFS) types 1 or 2 and <25% with BSFS types 6 or 7
- IBS with diarrhoea (IBS-D): >25% of bowel movements with BSFS types 6 or 7 and <25% with BSFS types 1 or 2
- IBS mixed (IBS-M): >25% of bowel movements with BSFS types 1 or 2 and >25% with BSFS types 6 or 7
- Unclassified IBS (IBS-U): An unclassified subcategory that meets criteria for IBS, but bowel movements cannot accurately be categorised into 1 of the 3 subgroups
- More than 10% of infectious enteritis develops into post-infection IBS (PI-IBS) (15).
The symptoms of each subtype overlap and the main symptoms of the patients change periodically, resulting in conversion between each subtype (16).
The Bristol Stool Form Scale is now available as an app for tracking.
Risk factors and comorbidities
Comorbidity is one of the characteristics of IBS. Many other psychosocial, biological, and environmental factors are associated with IBS and might influence symptom severity (8):
- Peripheral factors: Food, acute GI infection, mucosal inflammation, abdominal or pelvic surgery, menses.
- Central factors: Life stress, somatisation
 , anxiety or depression, poor coping skills, poor social support, maladaptive cognitions, abuse.
, anxiety or depression, poor coping skills, poor social support, maladaptive cognitions, abuse.
Post-infection IBS
The most well-recognised risk factor for IBS, observed in approximately 10% of patients, is previous acute enteric infection (17). PI-IBS can occur after bacterial, viral, or protozoal infections. The odds of developing IBS is increased 4-fold in exposed individuals 12 months after infection (15). Risk factors for PI-IBS development include female sex, exposure to antibiotics, psychological distress preceding the illness, and severity of infection (15). The prognosis might be better in patients with PI-IBS than in individuals with a non-infectious cause; however, one longitudinal follow-up study showed that 15% of patients with PI-IBS remained symptomatic 8 years later (18).
Role of Diet in the pathophysiology of IBS
Diet induces IBS symptoms via several mechanisms, including intestinal distention, direct chemical stimulation of the epithelial cells lining the intestinal lumen, and altered intestinal microbiota. There is no evidence that IBS patients suffer from a food allergy mediated by immunoglobulin E (19,20,21,22,23). It has also been documented that food intolerance does not occur in IBS, which is a non-toxic, non-immune-mediated reaction to bioactive chemicals in food (20,24).
Sex-specific factors
IBS often presents differently in males and females.
- The prevalence of IBS is 1.67 times higher in females than in males (25).
- Males and females appear to experience different symptoms to be the most bothersome and may react differently to treatment.
- IBS-C is significantly more common in females, whereas IBS-D is more common in males (26).
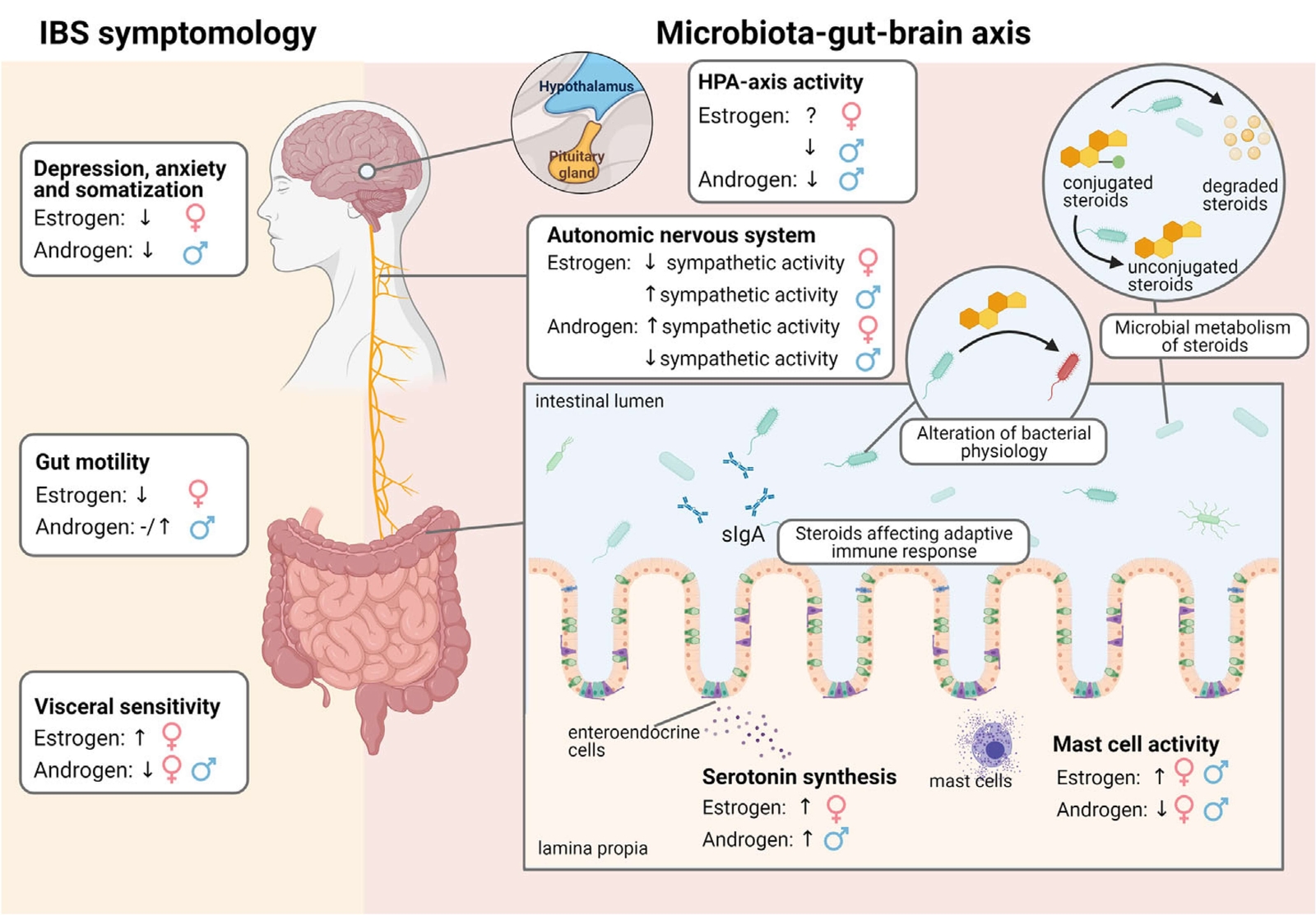
- Differences between males and females in GI tract motility and sensation, effects of early life and acute stress, hypothalamus-pituitary-adrenal (HPA) axis and autonomic responses, sex steroids and serotonergic pathways might explain some of these differences.
- It is becoming evident that the gut microbiota differs in males and females. This sexually dysmorphic microbiome has been termed the “microgenderome” (27).
- Sex can be a determining factor in the effectiveness of IBS treatment (5,28,29).
Figure 1 Effect of oestrogens and androgens on IBS symptomology and the microbiota-gut-brain axis in males and females (29) CC BY
Other risk factors/comorbidities
- Functional dyspepsia (FD) frequently overlaps with IBS. The pathogenesis of both IBS and FD is complicated and multifactorial, in which infectious or non-infectious inflammation and local or systemic immune response play significant roles (30).
- Compared with healthy control patients, patients with IBS were approximately 3-fold more likely to have anxiety, especially in female (48% prevalence) compared to male patients (32% prevalence) (31). The severity of anxiety correlates with some IBS symptoms in female but not in male IBS patients. It is possible that the greater anxiety and depression of females alters central pain processing and predisposes them to more severe GI tract symptoms (32).
- Helicobacter pylori infection may have a positive effect on the development of IBS in some populations (e.g. Asian populations) (33).
- IBS is more common in patients with functional somatic syndromes, such as fibromyalgia and chronic fatigue (34,35).
- A bidirectional association exists between IBS and restless legs syndrome (RLS), with a 2.6-fold increased odds of RLS in patients with IBS and a 3.87-fold increased odds of IBS in patients with RLS (36). The mechanism underlying this comorbidity is unclear; however, small intestinal bacterial overgrowth (SIBO), mast cell activation, pain sensory abnormalities, iron deficiency, and vitamin D metabolism have been proposed as possible mechanisms.
- Epidemiological evidence indicates a link between endometriosis and IBS, with a 3-fold higher risk of IBS in women with endometriosis compared to women without the condition (37). The sharing of symptoms and chronic inflammation means that endometriosis and IBS may coexist and be misdiagnosed, leading to delays in diagnosis, mismanagement, and unnecessary testing.
- Individuals who suffer from migraines have a significantly higher (2.5-fold) prevalence of IBS than non-migraine sufferers (38).
- Exposure to food additives could induce dysbiosis and dysregulation of gut homeostasis with an alteration of the gut barrier and activation of the immune response. These microbial changes could exacerbate IBS GI symptoms (39). Note, some food additives (polyols) are excluded in the low FODMAP diet for IBS. Similarly, exposure to nanoparticles, through food consumption or industrial exposure, can alter the gut microbiome in ways that may be associated with IBS (40). Common exposure to nanoparticles occurs via their use as food colourants, anticaking agents, and in antimicrobial packaging and pharmaceutical drugs.
Role of the gut microbiota in IBS
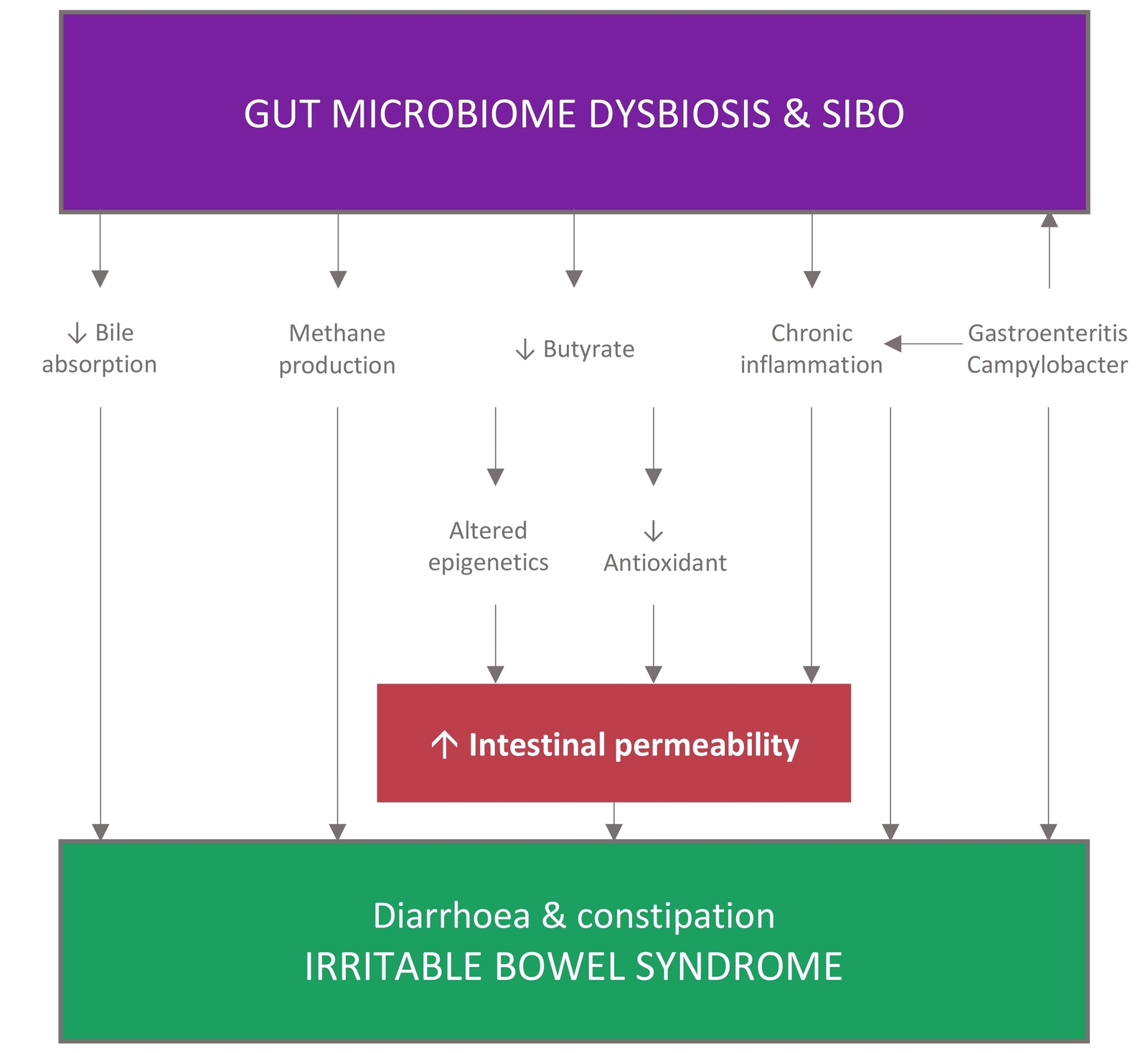
Studies worldwide provide convincing evidence that gut microbiota composition, diversity and stability alterations may be associated with IBS (41,42,43,44,45,46,47,48), indicating a critical role of gut microbiota in the pathogenesis of IBS. However, the causal relationship remains unclear. Interactions between the gut microbiota and host cells on the gut mucosal surface could affect the intestinal microenvironment. Conversely, an altered intestinal microenvironment could modify the gut microbiota (49).
- The severity of IBS symptoms is negatively correlated with microbial diversity (42,50,51), and the majority of IBS studies indicate an association between the loss of barrier function and symptoms such as abdominal pain and changes in the bowel function, particularly in IBS-D and PI-IBS (52).
- A proteolytic imbalance may cause GI inflammation (53,54). IBS is associated with high proteolytic activity
 (PA) which leads to intestinal barrier damage, increased permeability, and visceral hypersensitivity (42,55,56,57). The intestinal flora is a key regulator of PA (16).
(PA) which leads to intestinal barrier damage, increased permeability, and visceral hypersensitivity (42,55,56,57). The intestinal flora is a key regulator of PA (16). - Specific microbial strain changes may contribute to rapid GI transport, intestinal barrier dysfunction, congenital immune activation, and anxiety-like behaviour, all of which are common in IBS (16,58). For example, a reduction in Rhodobacillus coli may directly lead to short-chain fatty acids (SCFA) deficiency, ultimately resulting in the visceral pain of IBS (59).
- IBS is significantly associated with small intestinal bacterial overgrowth (SIBO) (51,60,61,62) and intestinal infection (60,63).
Figure 2 Theories on the gut microbiome physiologic mechanisms of IBS. Adapted from (62) CC BY
Gut-brain axis
IBS arises from dysfunctions of the gut-brain axis (1). Visceral hypersensitivity, altered GI permeability, immune dysfunction, or psychological disorder have been generally accepted as potential contributors to the pathogenesis of IBS. Evidence demonstrates that alterations in gut microbiota and dysregulation of the gut-brain axis may be associated with IBS pathogenesis through the production of metabolites such as SCFAs and the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) (64).
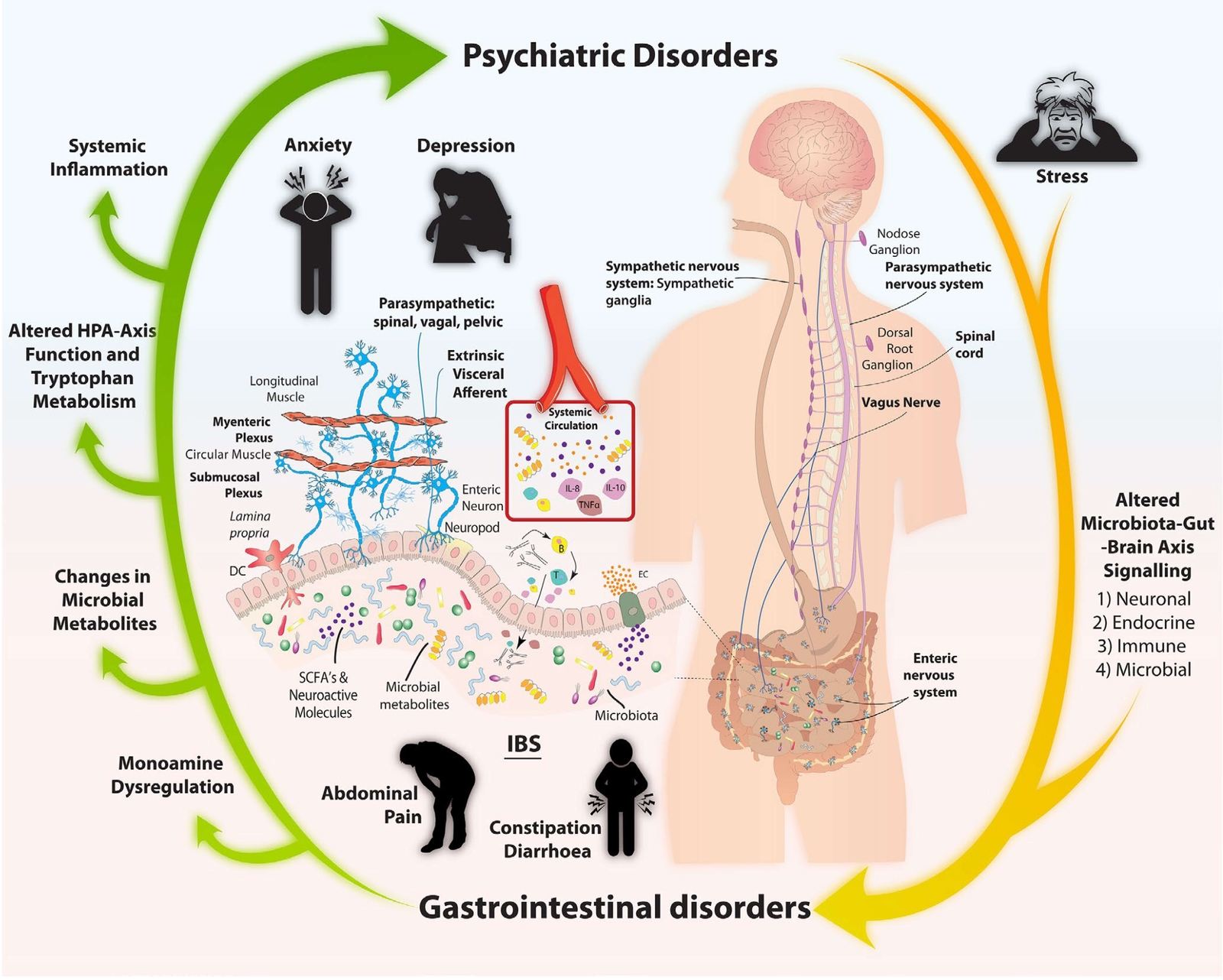
Figure 3 Summary of microbiota-gut-brain axis signalling pathways (65) CC BY
- In the gut-brain axis, 5-HT is associated with visceral hypersensitivity and GI permeability and may regulate GI motility, visceral sensing, and mucosal secretion by activating on both intrinsic excitatory and inhibitory enteric motor neurons (64).
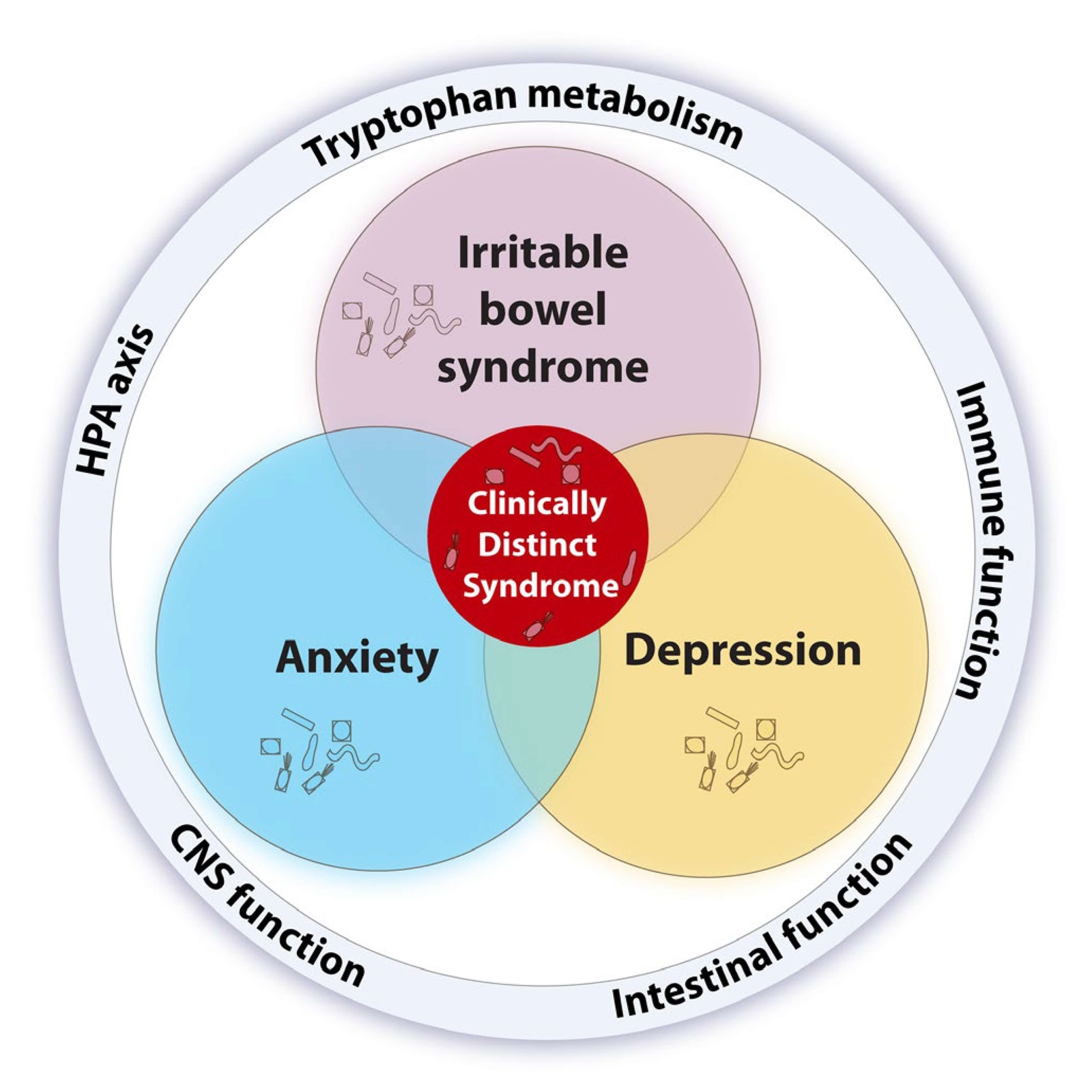
- Studies suggest that patients with either IBS, depression or anxiety, alone or comorbid, present with alterations in gut microbiota composition and harbour immune, endocrine, and serotonergic system alterations relevant to the common pathophysiology of these comorbid conditions (65).
- A gut signature composed of increased Bacteroides, Faecalibacterium and Lachnospiraceae is present in comorbid IBS and depression (66).
- It is still not known why specific microbiota configurations, compositional or functional, lead to IBS in some cases and IBS with psychiatric comorbidity in others (65).
Figure 4 A microbial perspective on the intersection between IBS, depression, and anxiety (65) CC BY