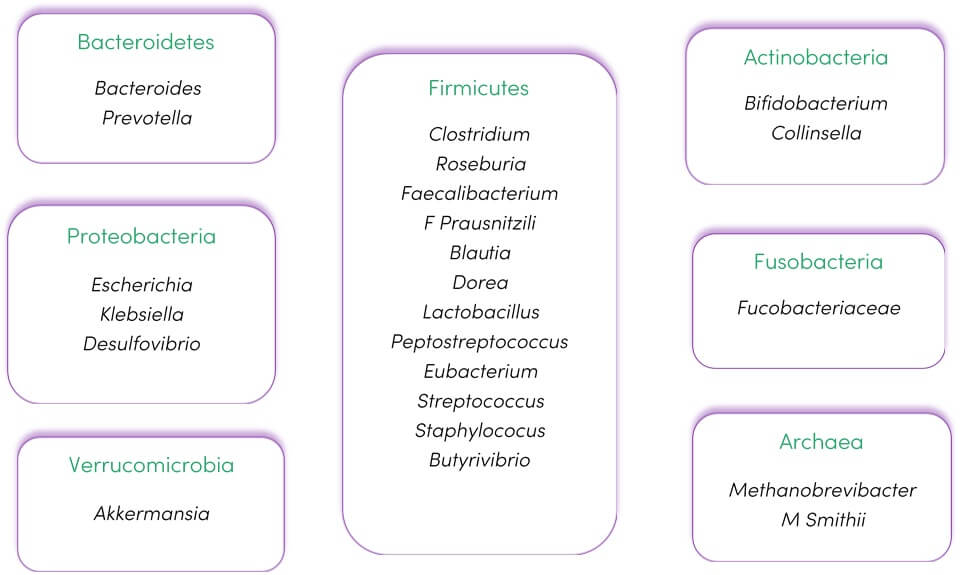
The human gut microbiome is a collection of tens of trillions of microorganisms populating the intestine. Bacteria are the predominant organisms, and most research is focused on them due to their high abundance and high diversity (see Figure 1). However, it also includes viruses, phages and fungi. The gut microbiota encompasses all microorganisms inhabiting the intestinal tract and their respective genomes (1).
The microbiome is a complex of interconnected ecological communities that communicate, cross-feed, recombine, and coevolve (2). The gut microbes perform a wide range of useful and health-promoting activities, however, they can also be responsible for the production of harmful molecules related to the development of several diseases (3).
Despite their omnipresence, centrality to life, and clear link to health and disease, we are only beginning to understand how microbes interact with each other and their hosts.
Figure 1. The predominant phyla and genera found in the intestinal microbiota. Adapted from (4) under CC BY 4.0
Traditional Understanding
Microbiome-based treatments have existed for many years and mostly include dietary recommendations and supplements from fermented milk products. These have evolved into the use of probiotics (5).
Latest Research
The complexity of the microbiota necessitates a movement from reductionist approaches that focus on individual pathogens in isolation to more holistic approaches that focus on interactions among members of the community and their hosts (2). The microbiome is recognised as an increasingly important component of disease prevention and treatment, more recently in the realm of chronic non-communicable diseases (6). Some of the current topics of interest and recent findings, outside of the traditional gastrointestinal disease association, include identification of:
Gut-brain axis
Gut microbiota can play an important role in the brain’s physiological, behavioural, and cognitive function. The microbiota has been implicated in the aetiology and progression of autism spectrum disorder, Parkinson’s, Alzheimer’s, behavioural impairment, anxiety and major depressive disorders (7,8,9)
Gut-lung axis
Microbial dysbiosis in the gut and the lung is increasingly being associated with the incidence and severity of asthma. The gut microbiota can influence immune responses at distant sites (such as the lung) via multiple mechanisms, including histamine release and immune effects of an increase in short chain fatty acids (SCFAs) (10,11)
Gut-liver axis
Growing evidence suggests a connection between alterations in the gut microbial composition and chronic liver diseases. The gut microbiota may modulate alcohol liver disease, non-alcoholic fatty liver disease, cirrhosis, and even hepatic carcinoma (12,13,14).
Gut-bone axis
The gut microbiota is responsible for bone physiology, regulating bone mass via the immune system and promoting bone resorption and formation via SCFA production (10)
Gut-vascular axis
Most cardiovascular disease (CVD) risk factors, including ageing, obesity, certain dietary patterns, and a sedentary lifestyle, have been shown to induce gut dysbiosis. Microbial components and metabolites may facilitate the development of CVD (15)
Gut-kidney axis
Microbiota metabolites influence chronic kidney disease progression, during which the kidney is no longer able to deal with microbial-derived uremic toxins (16)
The microbiome is also being studied in relation to the following areas of health:
- Cancer - Aetiology, progression and interaction with oncology treatment (12)
- Metabolic diseases - For example, obesity, diabetes (17,18,19)
- Cardiovascular disease – For example, the effect on lipid profiles and reducing hypertension (20,21,22,23)
- Immunomodulation - For example, the effect on vaccinations, allergy and autoimmune disease (11,24,25)
- Personalised nutrition – Individual differences in nutrient bioavailability, absorption, and even glycaemic response (26,27,28,29)
- Environmental impact - Environmental exposure, including smoking, impacts the microbiome, and the microbiome plays a role in metabolising environmental chemicals (30,31)
- Microbiota-drug interactions – The microbiota can affect drug metabolism and may alter drug efficacy (32). Similarly, medications (including non-antibiotics) can alter the microbiome (33)
- Systemic inflammation (11)
- Interaction with the human genome - The microbiome is associated with both host genetic variations and host gene expression(5). Host genetics have been shown to have a minor role in microbiome composition, with environmental factors having a greater impact (34)
- Lifestyle factors - For example, exercise, sleep deprivation, stress and weight loss (11,35,36,37)
- Circadian rhythms - The composition of the gut microbiome is influenced by circadian rhythm, which also then affects host circadian cycles and alters hormone regulation (11)
- Pancreatic diseases - Gut microbial dysbiosis is thought to contribute to the pathogenesis of pancreatic diseases such as pancreatitis and pancreatic cancer (38)
Treatment Options
Probiotics
Probiotics are live microorganisms which can boost the abundance of specific microbial species and may be consumed in fermented foods such as yoghurt or as capsulated supplements. Individual strain probiotic supplementation has been shown to be beneficial in many areas (15).
Delivering a single strain compared to a combination of bacteria in probiotic formulations is an important consideration, as each strain may have a different impact on microbial structure/function or on the host immune response (39,40). Circumstantial evidence supports the use of multistrain probiotics, particularly those with a high number of different strains. It’s possible that more strains convey more chances of success, a broader spectrum of efficacy and multistrain probiotics may provide additive or synergistic effects (41,42). Further research is required to confirm these effects.
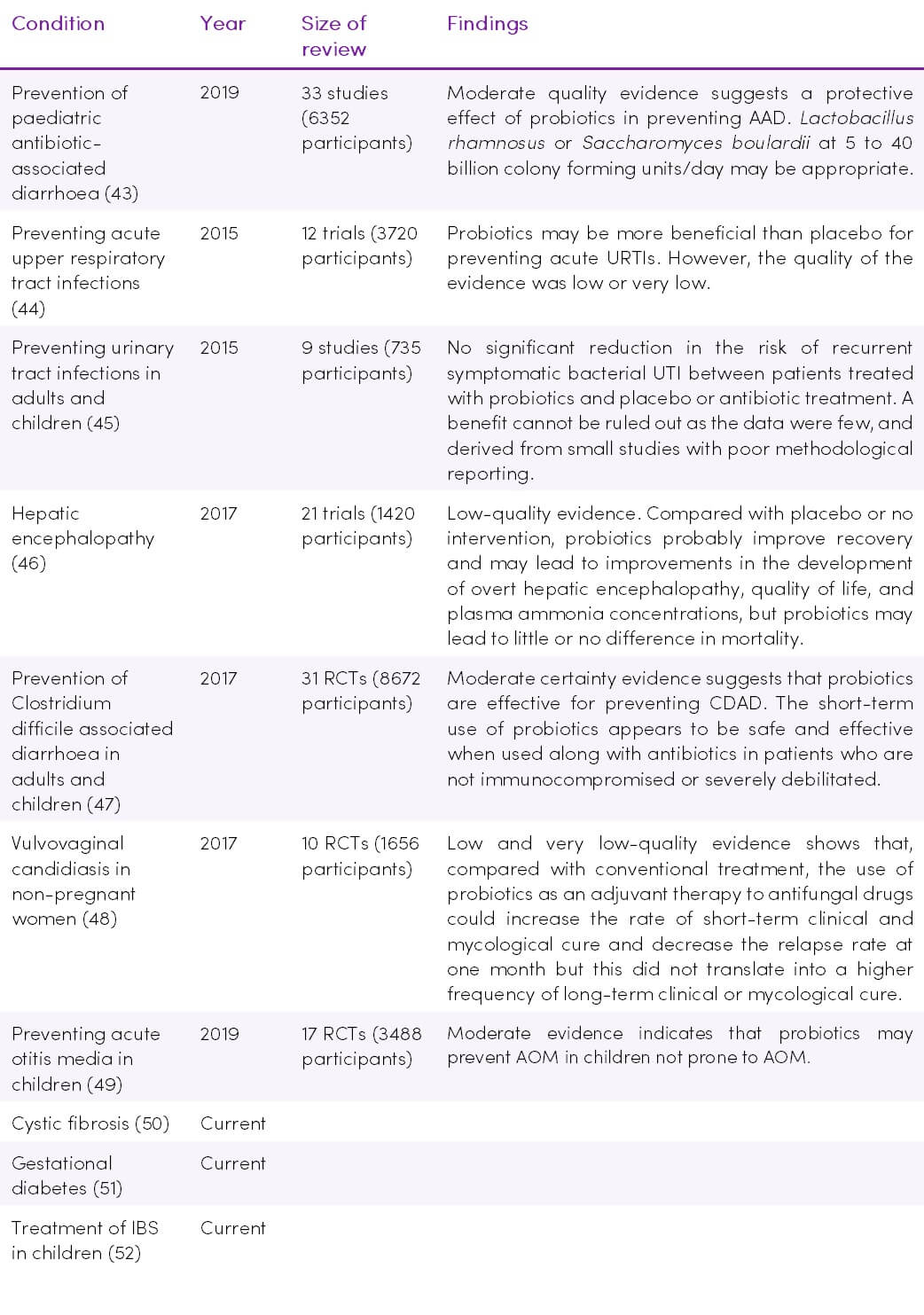
Table 1. Recent and current Cochrane Reviews on probiotics
Prebiotics
Prebiotics are non-digestible food ingredients such as dietary fibres and other digestion-resistant carbohydrates, which “feed” the microbiome. Carbohydrate fermentation by the gut microbiota produces SCFAs, which have wide-ranging physiological effects. Future trends may expand beyond fermentable substances to include novel prebiotics, such as bacteriophages, that can modulate the growth of certain bacteria and improve host health (15).
Psychobiotics
Psychobiotics are probiotics and prebiotics that have the potential to relieve neuropsychiatric symptoms, with compelling evidence for use in mental health (53).
Diet
Dietary effects on the human gut microbiome are rapid and driven by quality and quantity of dietary fat and carbohydrates (1). The typical Western diet may permanently reduce the capacity of the gut to support diversity.
Postbiotics/Paraprobiotics
Postbiotics refers to soluble factors (products or metabolic by-products) secreted by live bacteria or released after bacterial lysis. They have a clear chemical structure, safe dose parameters and a long shelf life which offer advantages over probiotics. Evidence shows that postbiotics possess antimicrobial, antioxidant, and immunomodulatory properties (54).
Paraprobiotics, also known as “non-viable probiotics”, refer to inactivated (non-viable) microbial cells, which, when administered in sufficient amounts, confer benefits to human health (54).
Faecal microbiota transplantation (FMT)
FMT is a procedure in which healthy donor faecal microbiota are introduced orally or through enemas or colonoscopy into recipient patients (1). They have become increasingly standardised products that are becoming acceptable to mainstream medicine. It has shown to be a promising treatment for recurring C. difficile infections, and current research is exploring its use in other areas of health, such as obesity, type 2 diabetes, inflammatory bowel diseases, non-alcoholic steatohepatitis, and recolonization after heavy antibiotic exposure (5,15).
Takeaway on the Microbiome
The vast body of evidence which is accumulating at a rapid pace is showing some very exciting trends in the field of the human gut microbiome and potential for disease prevention and treatment. Relatively cheap and easy to implement options through diet and probiotic/prebiotic supplements makes this a very promising area of clinical practice which can have an enormous impact on the lives of our clients.